Making an effective switch at the kinetochore by phosphorylation and dephosphorylation
- PMID: 23512483
- PMCID: PMC3665160
- DOI: 10.1007/s00412-013-0401-5
Making an effective switch at the kinetochore by phosphorylation and dephosphorylation
Abstract
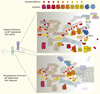
The kinetochore, the proteinaceous structure on the mitotic centromere, functions as a mechanical latch that hooks onto microtubules to support directional movement of chromosomes. The structure also brings in a number of signaling molecules, such as kinases and phosphatases, which regulate microtubule dynamics and cell cycle progression. Erroneous microtubule attachment is destabilized by Aurora B-mediated phosphorylation of multiple microtubule-binding protein complexes at the kinetochore, such as the KMN network proteins and the Ska/Dam1 complex, while Plk-dependent phosphorylation of BubR1 stabilizes kinetochore-microtubule attachment by recruiting PP2A-B56. Spindle assembly checkpoint (SAC) signaling, which is activated by unattached kinetochores and inhibits the metaphase-to-anaphase transition, depends on kinetochore recruitment of the kinase Bub1 through Mps1-mediated phosphorylation of the kinetochore protein KNL1 (also known as Blinkin in mammals, Spc105 in budding yeast, and Spc7 in fission yeast). Recruitment of protein phosphatase 1 to KNL1 is necessary to silence the SAC upon bioriented microtubule attachment. One of the key unsolved questions in the mitosis field is how a mechanical change at the kinetochore upon microtubule attachment is converted to these and other chemical signals that control microtubule attachment and the SAC. Rapid progress in the field is revealing the existence of an intricate signaling network created right on the kinetochore. Here we review the current understanding of phosphorylation-mediated regulation of kinetochore functions and discuss how this signaling network generates an accurate switch that turns on and off the signaling output in response to kinetochore-microtubule attachment.
Figures



References
-
- Abrieu A, Magnaghi-Jaulin L, Kahana JA, Peter M, Castro A, Vigneron S, Lorca T, Cleveland DW, Labbe JC. Mps1 is a kinetochore-associated kinase essential for the vertebrate mitotic checkpoint. Cell. 2001;106:83–93. - PubMed
-
- Ahonen LJ, Kallio MJ, Daum JR, Bolton M, Manke IA, Yaffe MB, Stukenberg PT, Gorbsky GJ. Polo-like kinase 1 creates the tension-sensing 3F3/2 phosphoepitope and modulates the association of spindle-checkpoint proteins at kinetochores. Curr Biol. 2005;15:1078–1089. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

