Inflammation and Hras signaling control epithelial-mesenchymal transition during skin tumor progression
- PMID: 23512660
- PMCID: PMC3613613
- DOI: 10.1101/gad.210427.112
Inflammation and Hras signaling control epithelial-mesenchymal transition during skin tumor progression
Abstract
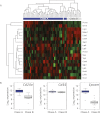
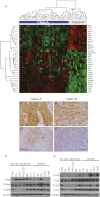
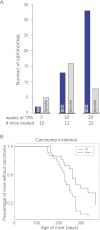
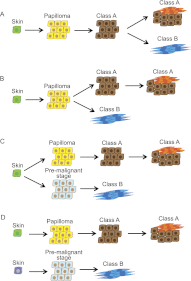
Epithelial-mesenchymal transition (EMT) is thought to be an important, possibly essential, component of the process of tumor dissemination and metastasis. About 20%-30% of Hras mutant mouse skin carcinomas induced by chemical initiation/promotion protocols have undergone EMT. Reduced exposure to TPA-induced chronic inflammation causes a dramatic reduction in classical papillomas and squamous cell carcinomas (SCCs), but the mice still develop highly invasive carcinomas with EMT properties, reduced levels of Hras and Egfr signaling, and frequent Ink4/Arf deletions. Deletion of Hras from the mouse germline also leads to a strong reduction in squamous tumor development, but tumors now acquire activating Kras mutations and exhibit more aggressive metastatic properties. We propose that invasive carcinomas can arise by different genetic and biological routes dependent on exposure to chronic inflammation and possibly from different target cell populations within the skin. Our data have implications for the use of inhibitors of inflammation or of Ras/Egfr pathway signaling for prevention or treatment of invasive cancers.
Figures


References
-
- Adorno M, Cordenonsi M, Montagner M, Dupont S, Wong C, Hann B, Solari A, Bobisse S, Rondina MB, Guzzardo V, et al. 2009. A mutant-p53/Smad complex opposes p63 to empower TGFβ-induced metastasis. Cell 137: 87–98 - PubMed
-
- Al-Hajj M, Clarke MF 2004. Self-renewal and solid tumor stem cells. Oncogene 23: 7274–7282 - PubMed
-
- Alam M, Ratner D 2001. Cutaneous squamous-cell carcinoma. N Engl J Med 344: 975–983 - PubMed
-
- Anastasi S, Fiorentino L, Fiorini M, Fraioli R, Sala G, Castellani L, Alema S, Alimandi M, Segatto O 2003. Feedback inhibition by RALT controls signal output by the ErbB network. Oncogene 22: 4221–4234 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
