One year outcomes in patients with acute lung injury randomised to initial trophic or full enteral feeding: prospective follow-up of EDEN randomised trial
- PMID: 23512759
- PMCID: PMC3601941
- DOI: 10.1136/bmj.f1532
One year outcomes in patients with acute lung injury randomised to initial trophic or full enteral feeding: prospective follow-up of EDEN randomised trial
Abstract
Objective: To evaluate the effect of initial low energy permissive underfeeding ("trophic feeding") versus full energy enteral feeding ("full feeding") on physical function and secondary outcomes in patients with acute lung injury.
Design: Prospective longitudinal follow-up evaluation of the NHLBI ARDS Clinical Trials Network's EDEN trial
Setting: 41hospitals in the United States.
Participants: 525 patients with acute lung injury.
Interventions: Randomised assignment to trophic or full feeding for up to six days; thereafter, all patients still receiving mechanical ventilation received full feeding.
Measurements: Blinded assessment of the age and sex adjusted physical function domain of the SF-36 instrument at 12 months after acute lung injury. Secondary outcome measures included survival; physical, psychological, and cognitive functioning; quality of life; and employment status at six and 12 months.
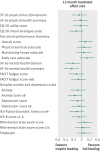
Results: After acute lung injury, patients had substantial physical, psychological, and cognitive impairments, reduced quality of life, and impaired return to work. Initial trophic versus full feeding did not affect mean SF-36 physical function at 12 months (55 (SD 33) v 55 (31), P=0.54), survival to 12 months (65% v 63%, P=0.63), or nearly all of the secondary outcomes.
Conclusion: In survivors of acute lung injury, there was no difference in physical function, survival, or multiple secondary outcomes at 6 and 12 month follow-up after initial trophic or full enteral feeding.
Trial registration: NCT No 00719446.
Trial registration: ClinicalTrials.gov NCT00719446.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures


References
-
- Barr J, Hecht M, Flavin KE, Khorana A, Gould MK. Outcomes in critically ill patients before and after the implementation of an evidence-based nutritional management protocol. Chest 2004;125:1446-57. - PubMed
-
- Rubinson L, Diette GB, Song X, Brower RG, Krishnan JA. Low caloric intake is associated with nosocomial bloodstream infections in patients in the medical intensive care unit. Crit Care Med 2004;32:350-7. - PubMed
-
- Ibrahim EH, Mehringer L, Prentice D, Sherman G, Schaiff R, Fraser V, et al. Early versus late enteral feeding of mechanically ventilated patients: results of a clinical trial. JPEN J Parenter Enteral Nutr 2002;26:174-81. - PubMed
-
- Krishnan JA, Parce PB, Martinez A, Diette GB, Brower RG. Caloric intake in medical ICU patients: consistency of care with guidelines and relationship to clinical outcomes. Chest 2003;124:297-305. - PubMed
-
- Dickerson RN, Boschert KJ, Kudsk KA, Brown RO. Hypocaloric enteral tube feeding in critically ill obese patients. Nutrition 2002;18:241-6. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
