Early-stage squamous cell carcinoma of the oropharynx: radiotherapy vs. trans-oral robotic surgery (ORATOR)--study protocol for a randomized phase II trial
- PMID: 23514246
- PMCID: PMC3621077
- DOI: 10.1186/1471-2407-13-133
Early-stage squamous cell carcinoma of the oropharynx: radiotherapy vs. trans-oral robotic surgery (ORATOR)--study protocol for a randomized phase II trial
Abstract
Background: The incidence of oropharyngeal squamous cell carcinoma (OPSCC) has markedly increased over the last three decades due to newly found associations with human papillomavirus (HPV) infection. Primary radiotherapy (RT) is the treatment of choice for OPSCC at most centers, and over the last decade, the addition of concurrent chemotherapy has led to a significant improvement in survival, but at the cost of increased acute and late toxicity. Transoral robotic surgery (TORS) has emerged as a promising alternative treatment, with preliminary case series demonstrating encouraging oncologic, functional, and quality of life (QOL) outcomes. However, comparisons of TORS and RT in a non-randomized fashion are susceptible to bias. The goal of this randomized phase II study is to compare QOL, functional outcomes, toxicity profiles, and survival following primary RT (± chemotherapy) vs. TORS (± adjuvant [chemo] RT) in patients with OPSCC.
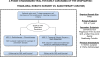
Methods/design: The target patient population comprises OPSCC patients who would be unlikely to require chemotherapy post-resection: Tumor stage T1-T2 with likely negative margins at surgery; Nodal stage N0-2, ≤3 cm in size, with no evidence of extranodal extension on imaging. Participants will be randomized in a 1:1 ratio between Arm 1 (RT ± chemotherapy) and Arm 2 (TORS ± adjuvant [chemo] RT). In Arm 1, patients with N0 disease will receive RT alone, whereas N1-2 patients will receive concurrent chemoradiation. In Arm 2, patients will undergo TORS along with selective neck dissections, which may be staged. Pathologic high-risk features will be used to determine the requirement for adjuvant radiotherapy +/- chemotherapy. The primary endpoint is QOL score using the M.D. Anderson Dysphagia Inventory (MDADI), with secondary endpoints including survival, toxicity, other QOL outcomes, and swallowing function. A sample of 68 patients is required.
Discussion: This study, if successful, will provide a much-needed randomized comparison of the conventional strategy of primary RT vs. the novel strategy of primary TORS. The trial is designed to provide a definitive QOL comparison between the two arms, and to inform the design of an eventual phase III trial for survival outcomes.
Trial registration: NCT01590355.
Figures
References
-
- Machtay M, Moughan J, Trotti A, Garden AS, Weber RS, Cooper JS, Forastiere A, Ang KK. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26(21):3582–3589. doi: 10.1200/JCO.2007.14.8841. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical