ERCC1 isoform expression and DNA repair in non-small-cell lung cancer
- PMID: 23514287
- PMCID: PMC4054818
- DOI: 10.1056/NEJMoa1214271
ERCC1 isoform expression and DNA repair in non-small-cell lung cancer
Abstract
Background: The excision repair cross-complementation group 1 (ERCC1) protein is a potential prognostic biomarker of the efficacy of cisplatin-based chemotherapy in non-small-cell lung cancer (NSCLC). Although several ongoing trials are evaluating the level of expression of ERCC1, no consensus has been reached regarding a method for evaluation.
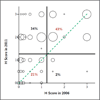
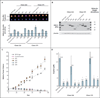
Methods: We used the 8F1 antibody to measure the level of expression of ERCC1 protein by means of immunohistochemical analysis in a validation set of samples obtained from 494 patients in two independent phase 3 trials (the National Cancer Institute of Canada Clinical Trials Group JBR.10 and the Cancer and Leukemia Group B 9633 trial from the Lung Adjuvant Cisplatin Evaluation Biology project). We compared the results of repeated staining of the entire original set of samples obtained from 589 patients in the International Adjuvant Lung Cancer Trial Biology study, which had led to the initial correlation between the absence of ERCC1 expression and platinum response, with our previous results in the same tumors. We mapped the epitope recognized by 16 commercially available ERCC1 antibodies and investigated the capacity of the different ERCC1 isoforms to repair platinum-induced DNA damage.
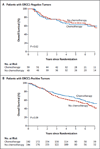
Results: We were unable to validate the predictive effect of immunostaining for ERCC1 protein. The discordance in the results of staining for ERCC1 suggested a change in the performance of the 8F1 antibody since 2006. We found that none of the 16 antibodies could distinguish among the four ERCC1 protein isoforms, whereas only one isoform produced a protein that had full capacities for nucleotide excision repair and cisplatin resistance.
Conclusions: Immunohistochemical analysis with the use of currently available ERCC1 antibodies did not specifically detect the unique functional ERCC1 isoform. As a result, its usefulness in guiding therapeutic decision making is limited. (Funded by Eli Lilly and others.).
Figures

References
-
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–3559. - PubMed
-
- Postel-Vinay S, Vanhecke E, Olaussen KA, Lord CJ, Ashworth A, Soria JC. The potential of exploiting DNA-repair defects for optimizing lung cancer treatment. Nat Rev Clin Oncol. 2012;9:144–155. - PubMed
-
- Bergstralh DT, Sekelsky J. Interstrand crosslink repair: can XPF-ERCC1 be let off the hook? Trends Genet. 2008;24:70–76. - PubMed
-
- Zheng Z, Chen T, Li X, Haura E, Sharma A, Bepler G. DNA synthesis and repair genes RRM1 and ERCC1 in lung cancer. N Engl J Med. 2007;356:800–808. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
