Four-year efficacy of RTS,S/AS01E and its interaction with malaria exposure
- PMID: 23514288
- PMCID: PMC5156295
- DOI: 10.1056/NEJMoa1207564
Four-year efficacy of RTS,S/AS01E and its interaction with malaria exposure
Abstract
Background: The candidate malaria vaccine RTS,S/AS01E has entered phase 3 trials, but data on long-term outcomes are limited.
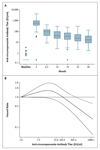
Methods: For 4 years, we followed children who had been randomly assigned, at 5 to 17 months of age, to receive three doses of RTS,S/AS01E vaccine (223 children) or rabies vaccine (224 controls). The end point was clinical malaria (temperature of ≥37.5°C and Plasmodium falciparum parasitemia density of >2500 parasites per cubic millimeter). Each child's exposure to malaria was estimated with the use of the distance-weighted local prevalence of malaria.
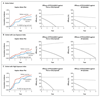
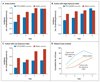
Results: Over a period of 4 years, 118 of 223 children who received the RTS,S/AS01E vaccine and 138 of 224 of the controls had at least 1 episode of clinical malaria. Vaccine efficacies in the intention-to-treat and per-protocol analyses were 29.9% (95% confidence interval [CI], 10.3 to 45.3; P=0.005) and 32.1% (95% CI, 11.6 to 47.8; P=0.004), respectively, calculated by Cox regression. Multiple episodes were common, with 551 and 618 malarial episodes in the RTS,S/AS01E and control groups, respectively; vaccine efficacies in the intention-to-treat and per-protocol analyses were 16.8% (95% CI, -8.6 to 36.3; P=0.18) and 24.3% (95% CI, 1.9 to 41.6; P=0.04), respectively, calculated by the Andersen-Gill extension of the Cox model. For every 100 vaccinated children, 65 cases of clinical malaria were averted. Vaccine efficacy declined over time (P=0.004) and with increasing exposure to malaria (P=0.001) in the per-protocol analysis. Vaccine efficacy was 43.6% (95% CI, 15.5 to 62.3) in the first year but was -0.4% (95% CI, -32.1 to 45.3) in the fourth year. Among children with a malaria-exposure index that was average or lower than average, the vaccine efficacy was 45.1% (95% CI, 11.3 to 66.0), but among children with a malaria-exposure index that was higher than average it was 15.9% (95% CI, -11.0 to 36.4).
Conclusions: The efficacy of RTS,S/AS01E vaccine over the 4-year period was 16.8%. Efficacy declined over time and with increasing malaria exposure. (Funded by the PATH Malaria Vaccine Initiative and Wellcome Trust; ClinicalTrials.gov number, NCT00872963.).
Figures
References
-
- World malaria report. Geneva: World Health Organization; 2011.
-
- The RTS,S Clinical Trials Partnership. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med. 2011;365:1863–75. - PubMed
-
- Olotu A, Lusingu J, Leach A, et al. Efficacy of RTS,S/AS01E malaria vaccine and exploratory analysis on anti-circumsporozoite antibody titres and protection in children aged 5-17 months in Kenya and Tanzania: a randomised controlled trial. Lancet Infect Dis. 2011;11:102–9. [Erratum, Lancet Infect Dis 2011;11:159.] - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
