Glycoprotein H and α4β1 integrins determine the entry pathway of alphaherpesviruses
- PMID: 23514881
- PMCID: PMC3648174
- DOI: 10.1128/JVI.03522-12
Glycoprotein H and α4β1 integrins determine the entry pathway of alphaherpesviruses
Abstract
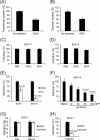
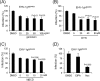
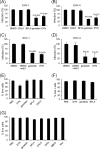
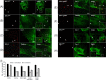
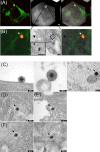
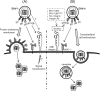
Herpesviruses enter cells either by direct fusion at the plasma membrane or from within endosomes, depending on the cell type and receptor(s). We investigated two closely related herpesviruses of horses, equine herpesvirus type 1 (EHV-1) and EHV-4, for which the cellular and viral determinants routing virus entry are unknown. We show that EHV-1 enters equine epithelial cells via direct fusion at the plasma membrane, while EHV-4 does so via an endocytic pathway, which is dependent on dynamin II, cholesterol, caveolin 1, and tyrosine kinase activity. Exchange of glycoprotein H (gH) between EHV-1 and EHV-4 resulted in rerouting of EHV-1 to the endocytic pathway, as did blocking of α4β1 integrins on the cell surface. Furthermore, a point mutation in the SDI integrin-binding motif of EHV-1 gH also directed EHV-1 to the endocytic pathway. Cumulatively, we show that viral gH and cellular α4β1 integrins are important determinants in the choice of alphaherpesvirus cellular entry pathways.
Figures




References
-
- Stein BS, Gowda SD, Lifson JD, Penhallow RC, Bensch KG, Engleman EG. 1987. pH-independent HIV entry into CD4-positive T cells via virus envelope fusion to the plasma membrane. Cell 49:659–668 - PubMed
-
- Mercer J, Schelhaas M, Helenius A. 2010. Virus entry by endocytosis. Annu. Rev. Biochem. 79:803–833 - PubMed
-
- Rahn E, Petermann P, Hsu MJ, Rixon FJ, Knebel-Morsdorf D. 2011. Entry pathways of herpes simplex virus type 1 into human keratinocytes are dynamin- and cholesterol-dependent. PLoS One 6:e25464 doi:10.1371/journal.pone.0025464 - DOI - PMC - PubMed
-
- Schelhaas M, Shah B, Holzer M, Blattmann P, Kuhling L, Day PM, Schiller JT, Helenius A. 2012. Entry of human papillomavirus type 16 by actin-dependent, clathrin- and lipid raft-independent endocytosis. PLoS Pathog. 8:e1002657 doi:10.1371/journal.ppat.1002657 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

