Deciphering the mechanism of defective interfering RNA (DI RNA) biogenesis reveals that a viral protein and the DI RNA act antagonistically in virus infection
- PMID: 23514891
- PMCID: PMC3648117
- DOI: 10.1128/JVI.03322-12
Deciphering the mechanism of defective interfering RNA (DI RNA) biogenesis reveals that a viral protein and the DI RNA act antagonistically in virus infection
Abstract
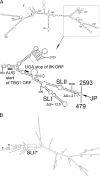
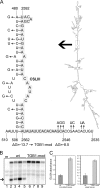
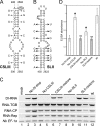
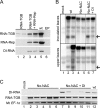
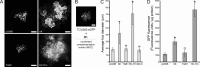
Potato mop-top virus (PMTV) produces a defective RNA (D RNA) encompassing the 5'-terminal 479 nucleotides (nt) and 3'-terminal 372 nt of RNA-TGB (where TGB is triple gene block). The mechanism that controls D RNA biogenesis and the role of D RNA in virus accumulation was investigated by introducing deletions, insertions, and point mutations into the sequences of the open reading frames (ORFs) of TGB1 and the 8-kilodalton (8K) protein that were identified as required for efficient production of the D RNA. Transient expression of RNA-TGB in the absence of RNA-Rep (which encodes the replicase) did not result in accumulation of D RNA, indicating that its production is dependent on PMTV replication. The D RNA could be eliminated by disrupting a predicted minus-strand stem-loop structure comprising complementary sequences of the 5' TGB1 ORF and the 3' 8K ORF, suggesting intramolecular template switching during positive-strand synthesis as a mechanism for the D RNA biogenesis. Virus accumulation was reduced when the 8K ORF was disrupted but D RNA was produced. Conversely, the virus accumulated at higher titers when the 8K ORF was intact and D RNA production was blocked. These data demonstrate that the D RNA interferes with virus infection and therefore should be referred to as a defective interfering RNA (DI RNA). The 8K protein was shown to be a weak silencing suppressor. This study provides an example of the interplay between a pathogen and its molecular parasite where virus accumulation was differentially regulated by the 8K protein and DI RNA, indicating that they play antagonistic roles and suggesting a mechanism by which the virus can attenuate replication, decreasing viral load and thereby enhancing its efficiency as a parasite.
Figures




Similar articles
-
A Stem-Loop Structure in Potato Leafroll Virus Open Reading Frame 5 (ORF5) Is Essential for Readthrough Translation of the Coat Protein ORF Stop Codon 700 Bases Upstream.J Virol. 2018 May 14;92(11):e01544-17. doi: 10.1128/JVI.01544-17. Print 2018 Jun 1. J Virol. 2018. PMID: 29514911 Free PMC article.
-
Defective interfering L RNA segments of tomato spotted wilt virus retain both virus genome termini and have extensive internal deletions.J Gen Virol. 1992 Oct;73 ( Pt 10):2509-16. doi: 10.1099/0022-1317-73-10-2509. J Gen Virol. 1992. PMID: 1402797
-
Stem-loop III in the 5' untranslated region is a cis-acting element in bovine coronavirus defective interfering RNA replication.J Virol. 2003 Jun;77(12):6720-30. doi: 10.1128/jvi.77.12.6720-6730.2003. J Virol. 2003. PMID: 12767992 Free PMC article.
-
Defective RNA Particles of Plant Viruses-Origin, Structure and Role in Pathogenesis.Viruses. 2022 Dec 16;14(12):2814. doi: 10.3390/v14122814. Viruses. 2022. PMID: 36560818 Free PMC article. Review.
-
Influenza virus DI particles: Defective interfering or delightfully interesting?PLoS Pathog. 2020 May 21;16(5):e1008436. doi: 10.1371/journal.ppat.1008436. eCollection 2020 May. PLoS Pathog. 2020. PMID: 32437428 Free PMC article. Review. No abstract available.
Cited by
-
Unveiling the biology of defective viral genomes in vitro and in vivo: implications for gene expression and pathogenesis of coronavirus.Virol J. 2023 Oct 6;20(1):225. doi: 10.1186/s12985-023-02189-7. Virol J. 2023. PMID: 37803357 Free PMC article.
-
Long Noncoding RNAs in Plant Viroids and Viruses: A Review.Pathogens. 2020 Sep 18;9(9):765. doi: 10.3390/pathogens9090765. Pathogens. 2020. PMID: 32961969 Free PMC article. Review.
-
Molecular and pathobiological characterization of 61 Potato mop-top virus full-length cDNAs reveals great variability of the virus in the centre of potato domestication, novel genotypes and evidence for recombination.Mol Plant Pathol. 2017 Aug;18(6):864-877. doi: 10.1111/mpp.12552. Epub 2017 May 11. Mol Plant Pathol. 2017. PMID: 28390168 Free PMC article.
-
Intact Viral Particle Counts Measured by Flow Virometry Provide Insight into the Infectivity and Genome Packaging Efficiency of Moloney Murine Leukemia Virus.J Virol. 2020 Jan 6;94(2):e01600-19. doi: 10.1128/JVI.01600-19. Print 2020 Jan 6. J Virol. 2020. PMID: 31694951 Free PMC article.
-
Acquisition of Full-Length Viral Helicase Domains by Insect Retrotransposon-Encoded Polypeptides.Front Microbiol. 2015 Dec 22;6:1447. doi: 10.3389/fmicb.2015.01447. eCollection 2015. Front Microbiol. 2015. PMID: 26733982 Free PMC article.
References
-
- Graves MV, Pogany J, Romero J. 1996. Defective interfering RNAs and defective viruses associated with multipartite RNA viruses of plants. Semin. Virol. 7:399–408
-
- Simon AE, Roossinck MJ, Havelda Z. 2004. Plant virus satellite and defective interfering RNAs: new paradigms for a new century. Annu. Rev. Phytopathol. 42:415–437 - PubMed
-
- Eliasco E, Livieratos IC, Müller G, Guzman M, Salazar LF, Coutts RH. 2006. Sequences of defective RNAs associated with potato yellow vein virus. Arch. Virol. 151:201–204 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

