Characteristics of the cellular receptor influence the intracellular fate and efficiency of virus infection
- PMID: 23514894
- PMCID: PMC3648180
- DOI: 10.1128/JVI.00398-13
Characteristics of the cellular receptor influence the intracellular fate and efficiency of virus infection
Abstract
The intracellular fate of internalized virus-receptor complexes is suspected of influencing the efficiency of virus infection. However, direct evidence of a link between infection and the fate of internalized virus has been difficult to obtain. To directly address this question, we generated human 293 cell lines stably expressing comparable cell surface levels of three different members of the somatostatin receptor family (SSTR) which have natural differences in intracellular trafficking. Utilizing a glycoprotein that recognizes SSTR, we found that distinctive receptor subtype-specific destinations correlated with observable differences in the level of infection. Infection via SSTR-2 and -3 is restricted at a point after receptor binding and endocytosis but prior to penetration into the host cytoplasm. In contrast, entry via SSTR-5 featured a slower internalization with greater dependence on cholesterol. Quantitative real-time PCR showed that virus bound to SSTR-5 was directed to an intracellular environment that allowed near-wild-type (WT) levels of penetration, possibly due to a more favorable complement of host cell proteases, whereas SSTR-2 and -3 directed virions to a degradative compartment in which cytosol penetration was less efficient. Taken together, the results support that the superior receptor capacity of SSTR-5 results from its internalization into a cellular compartment that is more favorable to the cytoplasmic penetration of viral cores and reverse transcription. They suggest that the intracellular destination of internalized complexes is an important characteristic of a virus receptor and may have exerted a selective pressure on the choice of an entry receptor during evolution of viral glycoproteins.
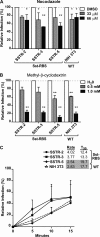
Figures


Similar articles
-
Targeted entry via somatostatin receptors using a novel modified retrovirus glycoprotein that delivers genes at levels comparable to those of wild-type viral glycoproteins.J Virol. 2012 Jan;86(1):373-81. doi: 10.1128/JVI.05411-11. Epub 2011 Oct 19. J Virol. 2012. PMID: 22013043 Free PMC article.
-
Differences in the internalization of self-inactivating VSVG-pseudotyped murine leukemia virus-based vectors in human and murine cells.J Virol Methods. 2018 May;255:14-22. doi: 10.1016/j.jviromet.2018.02.005. Epub 2018 Feb 6. J Virol Methods. 2018. PMID: 29425681
-
pH-independent murine leukemia virus ecotropic envelope-mediated cell fusion: implications for the role of the R peptide and p12E TM in viral entry.J Virol. 1994 May;68(5):3220-31. doi: 10.1128/JVI.68.5.3220-3231.1994. J Virol. 1994. PMID: 8151784 Free PMC article.
-
Dynamics of virus-receptor interactions in virus binding, signaling, and endocytosis.Viruses. 2015 Jun 2;7(6):2794-815. doi: 10.3390/v7062747. Viruses. 2015. PMID: 26043381 Free PMC article. Review.
-
Filovirus entry: a novelty in the viral fusion world.Viruses. 2012 Feb;4(2):258-75. doi: 10.3390/v4020258. Epub 2012 Feb 7. Viruses. 2012. PMID: 22470835 Free PMC article. Review.
Cited by
-
Comprehensive functional analysis of N-linked glycans on Ebola virus GP1.mBio. 2014 Jan 28;5(1):e00862-13. doi: 10.1128/mBio.00862-13. mBio. 2014. PMID: 24473128 Free PMC article.
-
Directed Molecular Evolution of an Engineered Gammaretroviral Envelope Protein with Dual Receptor Use Shows Stable Maintenance of Both Receptor Specificities.J Virol. 2015 Nov 25;90(3):1647-56. doi: 10.1128/JVI.02013-15. Print 2016 Feb 1. J Virol. 2015. PMID: 26608314 Free PMC article.
-
HIV-1 Tropism Determines Different Mutation Profiles in Proviral DNA.PLoS One. 2015 Sep 28;10(9):e0139037. doi: 10.1371/journal.pone.0139037. eCollection 2015. PLoS One. 2015. PMID: 26413773 Free PMC article.
References
-
- Coffin JM, Hughes SH, Varmus HE. 1997. Retroviruses. Cold Spring Harbor Laboratory Press, Plainview, NY - PubMed
-
- Anderson JL, Hope TJ. 2005. Intracellular trafficking of retroviral vectors: obstacles and advances. Gene Ther. 12:1667–1678 - PubMed
-
- Goff SP. 2007. Host factors exploited by retroviruses. Nat. Rev. Microbiol. 5:253–263 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

