Depletion of PAK1 enhances ubiquitin-mediated survivin degradation in pancreatic β-cells
- PMID: 23514967
- PMCID: PMC3662379
- DOI: 10.4161/isl.24029
Depletion of PAK1 enhances ubiquitin-mediated survivin degradation in pancreatic β-cells
Abstract
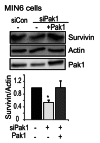
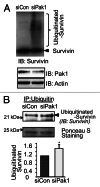
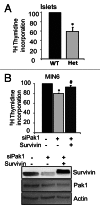
Functional β-cell mass deficiency in diabetes results from imbalanced β-cell death and replication, and decreased PAK1 protein levels in human islets from donors with type 2 diabetes implicates a possible role for PAK1 in maintaining β-cell mass. Here, we aim to address the linkage between PAK1 and Survivin, a protein essential for β-cell replication. PAK1 knockout (KO) mouse islets exhibited decreased expression of Survivin protein. MIN6 β-cells with siRNA-mediated suppression of PAK1 also had decreased Survivin protein and exhibited an increased level of ubiquitinated-Survivin. However, no significant changes in Survivin mRNA were found in islets from PAK1 KO mice and PAK1-depleted MIN6 β-cells. The decreased Survivin level in MIN6 cells subjected to hyperglycemic stress was prevented by expression of exogenous PAK1. Moreover, overexpressing Survivin restored proliferation of β-cells that was impaired by the loss of PAK1. These data implicate a role for PAK1 in regulating Survivin protein stability in the β-cell and suggest PAK1 as a potential molecular target for the restoration of β-cell mass.
Keywords: MIN6; PAK1; Survivin; Ubiquitination; mouse islet; pancreatic β-cell; replication.
Figures




Similar articles
-
Beta cell-specific PAK1 enrichment ameliorates diet-induced glucose intolerance in mice by promoting insulin biogenesis and minimising beta cell apoptosis.Diabetologia. 2025 Jan;68(1):152-165. doi: 10.1007/s00125-024-06286-2. Epub 2024 Oct 15. Diabetologia. 2025. PMID: 39404845 Free PMC article.
-
The Orphan Nuclear Receptor NR4A1 Protects Pancreatic β-Cells from Endoplasmic Reticulum (ER) Stress-mediated Apoptosis.J Biol Chem. 2015 Aug 21;290(34):20687-20699. doi: 10.1074/jbc.M115.654863. Epub 2015 Jul 8. J Biol Chem. 2015. PMID: 26157144 Free PMC article.
-
The p21-activated kinase (PAK1) is involved in diet-induced beta cell mass expansion and survival in mice and human islets.Diabetologia. 2016 Oct;59(10):2145-55. doi: 10.1007/s00125-016-4042-0. Epub 2016 Jul 9. Diabetologia. 2016. PMID: 27394663 Free PMC article.
-
EGF regulates survivin stability through the Raf-1/ERK pathway in insulin-secreting pancreatic β-cells.BMC Mol Biol. 2010 Aug 31;11:66. doi: 10.1186/1471-2199-11-66. BMC Mol Biol. 2010. PMID: 20807437 Free PMC article.
-
A role for PAK1 mediated phosphorylation of β-catenin Ser552 in the regulation of insulin secretion.Biochem J. 2021 Apr 30;478(8):1605-1615. doi: 10.1042/BCJ20200862. Biochem J. 2021. PMID: 33605402
Cited by
-
Signaling of the p21-activated kinase (PAK1) coordinates insulin-stimulated actin remodeling and glucose uptake in skeletal muscle cells.Biochem Pharmacol. 2014 Nov 15;92(2):380-8. doi: 10.1016/j.bcp.2014.08.033. Epub 2014 Sep 6. Biochem Pharmacol. 2014. PMID: 25199455 Free PMC article.
-
The Important Role of p21-Activated Kinases in Pancreatic Exocrine Function.Biology (Basel). 2025 Jan 22;14(2):113. doi: 10.3390/biology14020113. Biology (Basel). 2025. PMID: 40001881 Free PMC article. Review.
-
Beta cell-specific PAK1 enrichment ameliorates diet-induced glucose intolerance in mice by promoting insulin biogenesis and minimising beta cell apoptosis.Diabetologia. 2025 Jan;68(1):152-165. doi: 10.1007/s00125-024-06286-2. Epub 2024 Oct 15. Diabetologia. 2025. PMID: 39404845 Free PMC article.
-
Cepharanthine Enhances TRAIL-Mediated Apoptosis Through STAMBPL1-Mediated Downregulation of Survivin Expression in Renal Carcinoma Cells.Int J Mol Sci. 2018 Oct 22;19(10):3280. doi: 10.3390/ijms19103280. Int J Mol Sci. 2018. PMID: 30360403 Free PMC article.
-
Potential roles of PP2A-Rac1 signaling axis in pancreatic β-cell dysfunction under metabolic stress: Progress and promise.Biochem Pharmacol. 2020 Oct;180:114138. doi: 10.1016/j.bcp.2020.114138. Epub 2020 Jul 4. Biochem Pharmacol. 2020. PMID: 32634437 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
