CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia
- PMID: 23515080
- PMCID: PMC3742551
- DOI: 10.1126/scitranslmed.3005930
CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia
Abstract
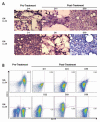
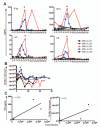
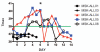
Adults with relapsed B cell acute lymphoblastic leukemia (B-ALL) have a dismal prognosis. Only those patients able to achieve a second remission with no minimal residual disease (MRD) have a hope for long-term survival in the context of a subsequent allogeneic hematopoietic stem cell transplantation (allo-HSCT). We have treated five relapsed B-ALL subjects with autologous T cells expressing a CD19-specific CD28/CD3ζ second-generation dual-signaling chimeric antigen receptor (CAR) termed 19-28z. All patients with persistent morphological disease or MRD(+) disease upon T cell infusion demonstrated rapid tumor eradication and achieved MRD(-) complete remissions as assessed by deep sequencing polymerase chain reaction. Therapy was well tolerated, although significant cytokine elevations, specifically observed in those patients with morphologic evidence of disease at the time of treatment, required lymphotoxic steroid therapy to ameliorate cytokine-mediated toxicities. Indeed, cytokine elevations directly correlated to tumor burden at the time of CAR-modified T cell infusions. Tumor cells from one patient with relapsed disease after CAR-modified T cell therapy, who was ineligible for additional allo-HSCT or T cell therapy, exhibited persistent expression of CD19 and sensitivity to autologous 19-28z T cell-mediated cytotoxicity, which suggests potential clinical benefit of additional CAR-modified T cell infusions. These results demonstrate the marked antitumor efficacy of 19-28z CAR-modified T cells in patients with relapsed/refractory B-ALL and the reliability of this therapy to induce profound molecular remissions, forming a highly effective bridge to potentially curative therapy with subsequent allo-HSCT.
Figures


References
-
- Fielding AK, Richards SM, Chopra R, Lazarus HM, Litzow MR, Buck G, Durrant IJ, Luger SM, Marks DI, Franklin IM, McMillan AK, Tallman MS, Rowe JM, Goldstone AH. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood. 2007;109:944. - PubMed
-
- Gokbuget N, Stanze D, Beck J, Diedrich H, Horst HA, Huttmann A, Kobbe G, Kreuzer KA, Leimer L, Reichle A, Schaich M, Schwartz S, Serve H, Starck M, Stelljes M, Stuhlmann R, Viardot A, Wendelin K, Freund M, Hoelzer D. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood. 2012;120:2032. - PubMed
-
- Bassan R, Spinelli O, Oldani E, Intermesoli T, Tosi M, Peruta B, Rossi G, Borlenghi E, Pogliani EM, Terruzzi E, Fabris P, Cassibba V, Lambertenghi-Deliliers G, Cortelezzi A, Bosi A, Gianfaldoni G, Ciceri F, Bernardi M, Gallamini A, Mattei D, Di Bona E, Romani C, Scattolin AM, Barbui T, Rambaldi A. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL) Blood. 2009;113:4153. - PubMed
-
- Brentjens RJ, Latouche JB, Santos E, Marti F, Gong MC, Lyddane C, King PD, Larson S, Weiss M, Riviere I, Sadelain M. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9:279. - PubMed
-
- Cooper LJ, Topp MS, Serrano LM, Gonzalez S, Chang WC, Naranjo A, Wright C, Popplewell L, Raubitschek A, Forman SJ, Jensen MC. T-cell clones can be rendered specific for CD19: toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood. 2003;101:1637. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

