Distinct extended amygdala circuits for divergent motivational states
- PMID: 23515155
- PMCID: PMC3778934
- DOI: 10.1038/nature12041
Distinct extended amygdala circuits for divergent motivational states
Abstract
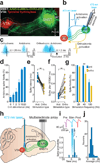
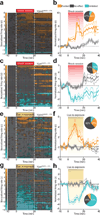
The co-morbidity of anxiety and dysfunctional reward processing in illnesses such as addiction and depression suggests that common neural circuitry contributes to these disparate neuropsychiatric symptoms. The extended amygdala, including the bed nucleus of the stria terminalis (BNST), modulates fear and anxiety, but also projects to the ventral tegmental area (VTA), a region implicated in reward and aversion, thus providing a candidate neural substrate for integrating diverse emotional states. However, the precise functional connectivity between distinct BNST projection neurons and their postsynaptic targets in the VTA, as well as the role of this circuit in controlling motivational states, have not been described. Here we record and manipulate the activity of genetically and neurochemically identified VTA-projecting BNST neurons in freely behaving mice. Collectively, aversive stimuli exposure produced heterogeneous firing patterns in VTA-projecting BNST neurons. By contrast, in vivo optically identified glutamatergic projection neurons displayed a net enhancement of activity to aversive stimuli, whereas the firing rate of identified GABAergic (γ-aminobutyric acid-containing) projection neurons was suppressed. Channelrhodopsin-2-assisted circuit mapping revealed that both BNST glutamatergic and GABAergic projections preferentially innervate postsynaptic non-dopaminergic VTA neurons, thus providing a mechanistic framework for in vivo circuit perturbations. In vivo photostimulation of BNST glutamatergic projections resulted in aversive and anxiogenic behavioural phenotypes. Conversely, activation of BNST GABAergic projections produced rewarding and anxiolytic phenotypes, which were also recapitulated by direct inhibition of VTA GABAergic neurons. These data demonstrate that functionally opposing BNST to VTA circuits regulate rewarding and aversive motivational states, and may serve as a crucial circuit node for bidirectionally normalizing maladaptive behaviours.
Figures



Comment in
-
Neuroscience: anxiety is the sum of its parts.Nature. 2013 Apr 11;496(7444):174-5. doi: 10.1038/nature12087. Epub 2013 Mar 20. Nature. 2013. PMID: 23515160 No abstract available.
References
-
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. - PubMed
-
- Nestler EJ, Carlezon WA., Jr. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. - PubMed
-
- Walker DL, Davis M. Role of the extended amygdala in short-duration versussustained fear: atribute to Dr Lennart Heimer. Brain Struct Funct. 2008;213:29–42. - PubMed
-
- Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. J Comp Neurol. 2005;490:270–294. - PubMed
Additional references
-
- Roitman MF, Wheeler RA, Carelli RM. Nucleus accumbens neurons are innately tuned for rewarding and aversive taste stimuli, encode their predictors, and are linked to motor output. Neuron. 2005;45:587–597. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 AA018610/AA/NIAAA NIH HHS/United States
- AA007573/AA/NIAAA NIH HHS/United States
- AA021043/AA/NIAAA NIH HHS/United States
- P60 AA011605/AA/NIAAA NIH HHS/United States
- R21 DA029325/DA/NIDA NIH HHS/United States
- AA011605/AA/NIAAA NIH HHS/United States
- P30 NS045892/NS/NINDS NIH HHS/United States
- T32 AA007573/AA/NIAAA NIH HHS/United States
- NS007431/NS/NINDS NIH HHS/United States
- F31 DA034472/DA/NIDA NIH HHS/United States
- DA032750/DA/NIDA NIH HHS/United States
- DA029325/DA/NIDA NIH HHS/United States
- DA034472/DA/NIDA NIH HHS/United States
- AA018610/AA/NIAAA NIH HHS/United States
- T32 NS007431/NS/NINDS NIH HHS/United States
- R01 DA032750/DA/NIDA NIH HHS/United States
- R37 DA032750/DA/NIDA NIH HHS/United States

