Construction of mesenchymal stem cell-containing collagen gel with a macrochanneled polycaprolactone scaffold and the flow perfusion culturing for bone tissue engineering
- PMID: 23515189
- PMCID: PMC3559226
- DOI: 10.1089/biores.2012.0234
Construction of mesenchymal stem cell-containing collagen gel with a macrochanneled polycaprolactone scaffold and the flow perfusion culturing for bone tissue engineering
Abstract
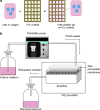
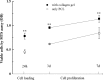
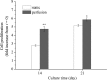
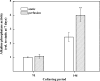
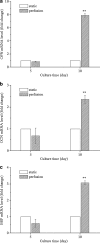
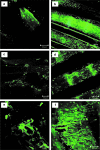
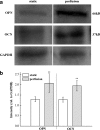
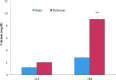
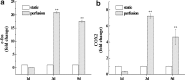
A novel bone tissue-engineering construct was developed by using poly(ɛ-caprolactone) (PCL)-macrochanneled scaffolds combined with stem cell-seeded collagen hydrogels and then applying flow perfusion culture. Rat mesenchymal stem cells (MSCs) were loaded into collagen hydrogels, which were then combined with macrochanneled PCL scaffolds. Collagen hydrogels were demonstrated to provide favorable growth environments for MSCs and to foster proliferation. Cell number determination identified retention of substantially fewer (50-60%) cells when they were seeded directly onto macrochanneled PCL than of cells engineered within collagen hydrogels. Additionally, the cells actively proliferated within the combined scaffold for up to 7 days. MSC-loaded collagen-PCL scaffolds were subsequently cultured under flow perfusion to promote proliferation and osteogenic differentiation. Cells proliferated to levels significantly higher in flow perfusion culture than that under static conditions during 21 days. A quantitative polymerase chain reaction (QPCR) assay revealed significant alterations in the transcription of bone-related genes such as osteopontin (OPN), osteocalcin (OCN), and bone sialoprotein (BSP), such as 8-, 2.5-, and 3-fold induction, respectively, after 10 days of flow perfusion relative to those in static culture. OPN and OCN protein levels, as determined by Western blot, increased under flow perfusion. Cellular mineralization was significantly enhanced by the flow perfusion during 21 and 28 days. Analyses of mechanosensitive gene expression induced by flow perfusion shear stress revealed significant upregulation of c-fos and cyclooxygenase-2 (COX-2) during the initial culture period (3-5 days), suggesting that osteogenic stimulation was possible as a result of mechanical force-driven transduction. These results provide valuable information for the design of a new bone tissue-engineering system by combining stem cell-loaded collagen hydrogels with macrochanneled scaffolds in flow perfusion culture.
Keywords: 3D scaffolds; bone tissue engineering; collagen gel; flow perfusion; osteogenic differentiation.
Figures


References
-
- Langer R. Vacanti JP. Tissue engineering. Science. 1993;260:920–926. - PubMed
-
- Laurencin CT. Ambrosis AM. Borden MD, et al. Tissue engineering: orthopedic applications. Annu Rev Biomed Eng. 1999;1:19–46. - PubMed
-
- Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4:518–524. - PubMed
-
- Liu X. Ma PX. Polymeric scaffolds for bone tissue engineering. Ann Biomed Eng. 2004;32:477–486. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
