A standardized rat model of volumetric muscle loss injury for the development of tissue engineering therapies
- PMID: 23515319
- PMCID: PMC3559228
- DOI: 10.1089/biores.2012.0271
A standardized rat model of volumetric muscle loss injury for the development of tissue engineering therapies
Abstract
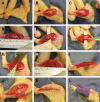
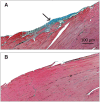
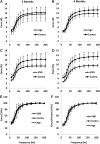
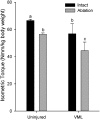
Soft tissue injuries involving volumetric muscle loss (VML) are defined as the traumatic or surgical loss of skeletal muscle with resultant functional impairment and represent a challenging clinical problem for both military and civilian medicine. In response, a variety of tissue engineering and regenerative medicine treatments are under preclinical development. A wide variety of animal models are being used, all with critical limitations. The objective of this study was to develop a model of VML that was reproducible and technically uncomplicated to provide a standardized platform for the development of tissue engineering and regenerative medicine solutions to VML repair. A rat model of VML involving excision of ∼20% of the muscle's mass from the superficial portion of the middle third of the tibialis anterior (TA) muscle was developed and was functionally characterized. The contralateral TA muscle served as the uninjured control. Additionally, uninjured age-matched control rats were also tested to determine the effect of VML on the contralateral limb. TA muscles were assessed at 2 and 4 months postinjury. VML muscles weighed 22.7% and 19.5% less than contralateral muscles at 2 and 4 months postinjury, respectively. These differences were accompanied by a reduction in peak isometric tetanic force (Po) of 28.4% and 32.5% at 2 and 4 months. Importantly, Po corrected for differences in body weight and muscle wet weights were similar between contralateral and age-matched control muscles, indicating that VML did not have a significant impact on the contralateral limb. Lastly, repair of the injury with a biological scaffold resulted in rapid vascularization and integration with the wound. The technical simplicity, reliability, and clinical relevance of the VML model developed in this study make it ideal as a standard model for the development of tissue engineering solutions for VML.
Keywords: extracellular matrix; fibrosis; muscle injury; muscle regeneration; regenerative medicine; tissue engineering.
Figures


References
-
- Grogan BF. Hsu JR. Volumetric muscle loss. J Am Acad Orthop Surg. 2011;19(Suppl 1):S35–37. - PubMed
-
- MacKenzie EJ. Bosse MJ. Kellam JF, et al. Characterization of patients with high-energy lower extremity trauma. J Orthop Trauma. 2000;14:455–466. - PubMed
-
- Grogan B. Hsu JR. Volumetric muscle loss. J Am Acad Orthop Surg. 2011;19(Suppl. 1):S35–S39. - PubMed
-
- Owens BD. Kragh JF., Jr Wenke JC, et al. Combat wounds in operation Iraqi Freedom and operation Enduring Freedom. J Trauma. 2008;64:295–299. - PubMed
-
- Lew TA. Walker JA. Wenke JC, et al. Characterization of craniomaxillofacial battle injuries sustained by United States service members in the current conflicts of Iraq and Afghanistan. J Oral Maxillofac Surg. 2010;68:3–7. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
