Integrin-dependent force transmission to the extracellular matrix by α-actinin triggers adhesion maturation
- PMID: 23515331
- PMCID: PMC3625291
- DOI: 10.1073/pnas.1220723110
Integrin-dependent force transmission to the extracellular matrix by α-actinin triggers adhesion maturation
Abstract
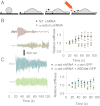
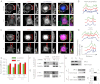
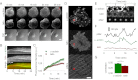
Focal adhesions are mechanosensitive elements that enable mechanical communication between cells and the extracellular matrix. Here, we demonstrate a major mechanosensitive pathway in which α-actinin triggers adhesion maturation by linking integrins to actin in nascent adhesions. We show that depletion of the focal adhesion protein α-actinin enhances force generation in initial adhesions on fibronectin, but impairs mechanotransduction in a subsequent step, preventing adhesion maturation. Expression of an α-actinin fragment containing the integrin binding domain, however, dramatically reduces force generation in depleted cells. This behavior can be explained by a competition between talin (which mediates initial adhesion and force generation) and α-actinin for integrin binding. Indeed, we show in an in vitro assay that talin and α-actinin compete for binding to β3 integrins, but cooperate in binding to β1 integrins. Consistently, we find opposite effects of α-actinin depletion and expression of mutants on substrates that bind β3 integrins (fibronectin and vitronectin) versus substrates that only bind β1 integrins (collagen). We thus suggest that nascent adhesions composed of β3 integrins are initially linked to the actin cytoskeleton by talin, and then α-actinin competes with talin to bind β3 integrins. Force transmitted through α-actinin then triggers adhesion maturation. Once adhesions have matured, α-actinin recruitment correlates with force generation, suggesting that α-actinin is the main link transmitting force between integrins and the cytoskeleton in mature adhesions. Such a multistep process enables cells to adjust forces on matrices, unveiling a role of α-actinin that is different from its well-studied function as an actin cross-linker.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Paszek MJ, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

