Characteristics of lung cancers harboring NRAS mutations
- PMID: 23515407
- PMCID: PMC3643999
- DOI: 10.1158/1078-0432.CCR-12-3173
Characteristics of lung cancers harboring NRAS mutations
Abstract
Purpose: We sought to determine the frequency and clinical characteristics of patients with lung cancer harboring NRAS mutations. We used preclinical models to identify targeted therapies likely to be of benefit against NRAS-mutant lung cancer cells.
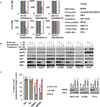
Experimental design: We reviewed clinical data from patients whose lung cancers were identified at six institutions or reported in the Catalogue of Somatic Mutations in Cancer (COSMIC) to harbor NRAS mutations. Six NRAS-mutant cell lines were screened for sensitivity against inhibitors of multiple kinases (i.e., EGFR, ALK, MET, IGF-1R, BRAF, PI3K, and MEK).
Results: Among 4,562 patients with lung cancers tested, NRAS mutations were present in 30 (0.7%; 95% confidence interval, 0.45%-0.94%); 28 of these had no other driver mutations. 83% had adenocarcinoma histology with no significant differences in gender. While 95% of patients were former or current smokers, smoking-related G:C>T:A transversions were significantly less frequent in NRAS-mutated lung tumors than KRAS-mutant non-small cell lung cancer [NSCLC; NRAS: 13% (4/30), KRAS: 66% (1772/2733), P < 0.00000001]. Five of 6 NRAS-mutant cell lines were sensitive to the MEK inhibitors, selumetinib and trametinib, but not to other inhibitors tested.
Conclusion: NRAS mutations define a distinct subset of lung cancers (∼1%) with potential sensitivity to MEK inhibitors. Although NRAS mutations are more common in current/former smokers, the types of mutations are not those classically associated with smoking.
©2013 AACR.
Conflict of interest statement
There are no other conflicts to report.
Figures

References
-
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004 May 20;350(21):2129–2139. - PubMed
-
- Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004 Jun 4;304(5676):1497–1500. - PubMed
-
- Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010 Jun 24;362(25):2380–2388. - PubMed
-
- Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010 Feb;11(2):121–128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U54 CA143798/CA/NCI NIH HHS/United States
- K12 CA090625/CA/NCI NIH HHS/United States
- P30 CA046934/CA/NCI NIH HHS/United States
- RC2-CA148394/CA/NCI NIH HHS/United States
- P30-CA68485/CA/NCI NIH HHS/United States
- R01 CA121210/CA/NCI NIH HHS/United States
- P50 CA090949/CA/NCI NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- RC2 CA148394/CA/NCI NIH HHS/United States
- K23 CA149079/CA/NCI NIH HHS/United States
- U54-CA143798/CA/NCI NIH HHS/United States
- R01-CA121210/CA/NCI NIH HHS/United States
- CA90949/CA/NCI NIH HHS/United States
- P01-CA129243/CA/NCI NIH HHS/United States
- P01 CA129243/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

