The energy cost of action potential propagation in dopamine neurons: clues to susceptibility in Parkinson's disease
- PMID: 23515615
- PMCID: PMC3600574
- DOI: 10.3389/fncom.2013.00013
The energy cost of action potential propagation in dopamine neurons: clues to susceptibility in Parkinson's disease
Abstract
Dopamine neurons of the substantia nigra pars compacta (SNc) are uniquely sensitive to degeneration in Parkinson's disease (PD) and its models. Although a variety of molecular characteristics have been proposed to underlie this sensitivity, one possible contributory factor is their massive, unmyelinated axonal arbor that is orders of magnitude larger than other neuronal types. We suggest that this puts them under such a high energy demand that any stressor that perturbs energy production leads to energy demand exceeding supply and subsequent cell death. One prediction of this hypothesis is that those dopamine neurons that are selectively vulnerable in PD will have a higher energy cost than those that are less vulnerable. We show here, through the use of a biology-based computational model of the axons of individual dopamine neurons, that the energy cost of axon potential propagation and recovery of the membrane potential increases with the size and complexity of the axonal arbor according to a power law. Thus SNc dopamine neurons, particularly in humans, whose axons we estimate to give rise to more than 1 million synapses and have a total length exceeding 4 m, are at a distinct disadvantage with respect to energy balance which may be a factor in their selective vulnerability in PD.
Keywords: Parkinson's disease; axons; dopamine; energy metabolism; neurodegeneration; nigrostriatal pathway; unmyelinated.
Figures


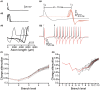



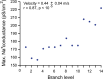

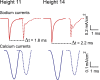
References
-
- Aisley A. J. (2001). The Physiology of Excitable Cells. Cambridge: Cambridge University Press
-
- Amini B., Clark J. W., Canavier C. C. (1999). Calcium dynamics underlying pacemaker-like and burst firing oscillations in midbrain dopaminergic neurons: a computational study. J. Neurophysiol. 82, 2249–2261 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

