Metal-organic frameworks constructed from monomeric, dimeric and trimeric phenanthroline citrate zinc building units
- PMID: 23516124
- PMCID: PMC3600651
- DOI: 10.1016/j.inoche.2009.04.028
Metal-organic frameworks constructed from monomeric, dimeric and trimeric phenanthroline citrate zinc building units
Abstract
Adduct of mononuclear and dinuclear citrate zinc complex [Zn(Hcit)(phen)(H2O)][Zn2(Hcit)(phen)2(H2O)3]·13.5H2O (1) and its aggregate [Zn3(Hcit)2(phen)4]n·14nH2O (2) (H4cit = citric acid, phen = 1,10-phenanthroline) were synthesized in weak acidic solutions. The former was obtained from the reaction of zinc nitrate, citric acid and phenanthroline in a molar ratio of 3 : 2 : 3, while a slightly excess of phenanthroline results in the formation of the polymeric product 2 in a molar ratio of 3 : 2 : 4. Transformation of 1 to 2 was finished by the reaction of 1 with an equimolar of phenanthroline in 72% yield. Reverse conversion of 2 to 1 is obtained in 77% yield, showing an equilibrium between 1 and 2. Neutral compound 1 consists of one monomeric anionic unit [Zn(Hcit)(phen)(H2O)]- and one dimeric cationic unit [Zn2(Hcit)(phen)2(H2O)3]+ that connect each other by strong hydrogen bonds [O6⋯O4w 2.636(2); O7⋯O3w 2.630(3) Å]. In 2, the citrate ligand links each trinuclear unit [Zn3(Hcit)2(phen)4] to generate an infinite 1D chain that extents into a 3D supramolecular structure by intra- and inter-molecular hydrogen bonds. Moreover, 1 and 2 exhibit strong fluorescence at room temperature.
Keywords: Citric acid; Crystal structure; Interconversion; Mixed ligands; Zinc.
Figures



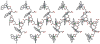

References
-
- Rao CNR, Natarajan S, Vaidhyanathan R. Angew Chem, Int Ed. 2004;43:1466. - PubMed
-
- Chu Q, Liu GX, Huang YQ, Wang XF, Sun WY. Dalton Trans. 2007:4302. - PubMed
-
- Blay G, Fernandez I, Gimenez T, Pedro JR, Ruiz R, Prdo E, Lloret F, Munoz MC. Chem Commun. 2001:2102. - PubMed
-
- Lassahn PG, Lozan V, Timco GA, Christian P, Janiak C, Winpenny REP. J Catal. 2004;222:260.
-
- Hong MC, Shako YJ, Su WP, Cao R, Fujita M, Zhou ZY, Chan ASC. Angew Chem, Int Ed. 2000;39:2468. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
