Protein aggregation and degradation mechanisms in neurodegenerative diseases
- PMID: 23516262
- PMCID: PMC3601466
Protein aggregation and degradation mechanisms in neurodegenerative diseases
Abstract
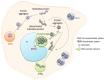
Neurodegenerative diseases are characterized by selective neuronal vulnerability and neurodegeneration in specific brain regions. The pathogenesis of these disorders centrally involves abnormal accumulation and aggregation of specific proteins, which are deposited in intracellular inclusions or extracellular aggregates that are characteristic for each disease. Increasing evidence suggests that genetic mutations or environmental factors can instigate protein misfolding and aggregation in these diseases. Consequently, neurodegenerative diseases are often considered as conformational diseases. This idea is further supported by studies implicating that impairment of the protein quality control (PQC) and clearance systems, such as the ubiquitin-proteasome system and autophagosome-lysosome pathway, may lead to the abnormal accumulation of disease-specific proteins. This suggests that similar pathological mechanisms may underlie the pathogenesis of the different neurodegenerative disorders. Interestingly, several proteins that are known to associate with neurodegenerative diseases have been identified as important regulators of PQC and clearance systems. In this review, we summarize the central features of abnormal protein accumulation in different common neurodegenerative diseases and discuss some aspects of specific disease-associated proteins regulating the PQC and clearance mechanisms, such as ubiquilin-1.
Keywords: IPOD; JUNQ; Protein quality control; aggresome; autophagy; inclusion body; neurodegenerative diseases; protein misfolding; ubiquilin-1; ubiquitin-proteasome system.
Figures


References
-
- Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci. 2003;4:49–60. - PubMed
-
- Ross CA, Poirier MA. Protein aggregation and neurodegenerative disease. Nat Med. 2004;10(Suppl):S10–7. - PubMed
-
- Schubert U, Anton LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–774. - PubMed
-
- Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. - PubMed
-
- Ciechanover A, Orian A, Schwartz AL. Ubiquitin-mediated proteolysis: biological regulation via destruction. Bioessays. 2000;22:442–451. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
