Calpains, cleaved mini-dysferlinC72, and L-type channels underpin calcium-dependent muscle membrane repair
- PMID: 23516275
- PMCID: PMC6704986
- DOI: 10.1523/JNEUROSCI.3560-12.2013
Calpains, cleaved mini-dysferlinC72, and L-type channels underpin calcium-dependent muscle membrane repair
Abstract
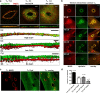
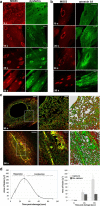
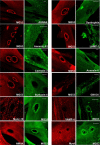
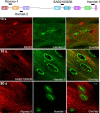
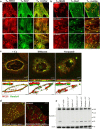
Dysferlin is proposed as a key mediator of calcium-dependent muscle membrane repair, although its precise role has remained elusive. Dysferlin interacts with a new membrane repair protein, mitsugumin 53 (MG53), an E3 ubiquitin ligase that shows rapid recruitment to injury sites. Using a novel ballistics assay in primary human myotubes, we show it is not full-length dysferlin recruited to sites of membrane injury but an injury-specific calpain-cleavage product, mini-dysferlinC72. Mini-dysferlinC72-rich vesicles are rapidly recruited to injury sites and fuse with plasma membrane compartments decorated by MG53 in a process coordinated by L-type calcium channels. Collective interplay between activated calpains, dysferlin, and L-type channels explains how muscle cells sense a membrane injury and mount a specialized response in the unique local environment of a membrane injury. Mini-dysferlinC72 and MG53 form an intricate lattice that intensely labels exposed phospholipids of injury sites, then infiltrates and stabilizes the membrane lesion during repair. Our results extend functional parallels between ferlins and synaptotagmins. Whereas otoferlin exists as long and short splice isoforms, dysferlin is subject to enzymatic cleavage releasing a synaptotagmin-like fragment with a specialized protein- or phospholipid-binding role for muscle membrane repair.
Figures


References
-
- Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, McNeil PL, Campbell KP. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423:168–172. - PubMed
-
- Bashir R, Britton S, Strachan T, Keers S, Vafiadaki E, Lako M, Richard I, Marchand S, Bourg N, Argov Z, Sadeh M, Mahjneh I, Marconi G, Passos-Bueno MR, Moreira Ede S, Zatz M, Beckmann JS, Bushby K. A gene related to Caenorhabditis elegans spermatogenesis factor fer-1 is mutated in limb-girdle muscular dystrophy type 2B. Nat Genet. 1998;20:37–42. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
