Dissociation of frontotemporal dementia-related deficits and neuroinflammation in progranulin haploinsufficient mice
- PMID: 23516300
- PMCID: PMC3740510
- DOI: 10.1523/JNEUROSCI.6103-11.2013
Dissociation of frontotemporal dementia-related deficits and neuroinflammation in progranulin haploinsufficient mice
Abstract
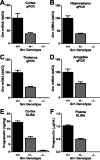
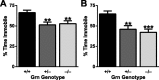
Frontotemporal dementia (FTD) is a neurodegenerative disease with hallmark deficits in social and emotional function. Heterozygous loss-of-function mutations in GRN, the progranulin gene, are a common genetic cause of the disorder, but the mechanisms by which progranulin haploinsufficiency causes neuronal dysfunction in FTD are unclear. Homozygous progranulin knock-out (Grn(-/-)) mice have been studied as a model of this disorder and show behavioral deficits and a neuroinflammatory phenotype with robust microglial activation. However, homozygous GRN mutations causing complete progranulin deficiency were recently shown to cause a different neurological disorder, neuronal ceroid lipofuscinosis, suggesting that the total absence of progranulin may have effects distinct from those of haploinsufficiency. Here, we studied progranulin heterozygous (Grn(+/-)) mice, which model progranulin haploinsufficiency. We found that Grn(+/-) mice developed age-dependent social and emotional deficits potentially relevant to FTD. However, unlike Grn(-/-) mice, behavioral deficits in Grn(+/-) mice occurred in the absence of gliosis or increased expression of tumor necrosis factor-α. Instead, we found neuronal abnormalities in the amygdala, an area of selective vulnerability in FTD, in Grn(+/-) mice. Our findings indicate that FTD-related deficits resulting from progranulin haploinsufficiency can develop in the absence of detectable gliosis and neuroinflammation, thereby dissociating microglial activation from functional deficits and suggesting an important effect of progranulin deficiency on neurons.
Figures





References
-
- Ahmed Z, Sheng H, Xu YF, Lin WL, Innes AE, Gass J, Yu X, Wuertzer CA, Hou H, Chiba S, Yamanouchi K, Leissring M, Petrucelli L, Nishihara M, Hutton ML, McGowan E, Dickson DW, Lewis J. Accelerated lipofuscinosis and ubiquitination in granulin knockout mice suggest a role for progranulin in successful aging. Am J Pathol. 2010;177:311–324. - PMC - PubMed
-
- Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 2004;431:805–810. - PubMed
-
- Bosch OJ, Neumann ID. Vasopressin released within the central amygdala promotes maternal aggression. Eur J Neurosci. 2010;31:883–891. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
