Mechanisms that link the oncogenic epithelial-mesenchymal transition to suppression of anoikis
- PMID: 23516327
- PMCID: PMC3603508
- DOI: 10.1242/jcs.120907
Mechanisms that link the oncogenic epithelial-mesenchymal transition to suppression of anoikis
Abstract
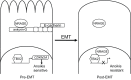
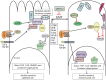
The oncogenic epithelial-mesenchymal transition (EMT) contributes to tumor progression in various context-dependent ways, including increased metastatic potential, expansion of cancer stem cell subpopulations, chemo-resistance and disease recurrence. One of the hallmarks of EMT is resistance of tumor cells to anoikis. This resistance contributes to metastasis and is a defining property not only of EMT but also of cancer stem cells. Here, we review the mechanistic coupling between EMT and resistance to anoikis. The discussion focuses on several key aspects. First, we provide an update on new pathways that lead from the loss of E-cadherin to anoikis resistance. We then discuss the relevance of transcription factors that are crucial in wound healing in the context of oncogenic EMT. Next, we explore the consequences of the breakdown of cell-polarity complexes upon anoikis sensitivity, through the Hippo, Wnt and transforming growth factor β (TGF-β) pathways, emphasizing points of crossregulation. Finally, we summarize the direct regulation of cell survival genes through EMT-inducing transcription factors, and the roles of the tyrosine kinases focal adhesion kinase (FAK) and TrkB neurotrophin receptor in EMT-related regulation of anoikis. Emerging from these studies are unifying principles that will lead to improvements in cancer therapy by reprogramming sensitivity of anoikis.
Figures

References
-
- Brabletz T., Jung A., Reu S., Porzner M., Hlubek F., Kunz–Schughart L. A., Knuechel R., Kirchner T. (2001). Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc. Natl. Acad. Sci. USA 98, 10356–10361 10.1073/pnas.171610498 - DOI - PMC - PubMed
-
- Canel M., Serrels A., Miller D., Timpson P., Serrels B., Frame M. C., Brunton V. G. (2010). Quantitative in vivo imaging of the effects of inhibiting integrin signaling via Src and FAK on cancer cell movement: effects on E-cadherin dynamics. Cancer Res. 70, 9413–9422 10.1158/0008-5472.CAN-10-1454 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

