The HPV16 E6 oncoprotein causes prolonged receptor protein tyrosine kinase signaling and enhances internalization of phosphorylated receptor species
- PMID: 23516367
- PMCID: PMC3597533
- DOI: 10.1371/journal.ppat.1003237
The HPV16 E6 oncoprotein causes prolonged receptor protein tyrosine kinase signaling and enhances internalization of phosphorylated receptor species
Abstract
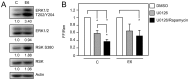
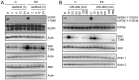
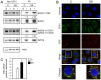
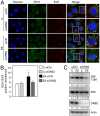
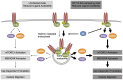
The high-risk human papillomavirus (HPV) E6 proteins are consistently expressed in HPV-associated lesions and cancers. HPV16 E6 sustains the activity of the mTORC1 and mTORC2 signaling cascades under conditions of growth factor deprivation. Here we report that HPV16 E6 activated mTORC1 by enhanced signaling through receptor protein tyrosine kinases, including epidermal growth factor receptor and insulin receptor and insulin-like growth factor receptors. This is evidenced by sustained signaling through these receptors for several hours after growth factor withdrawal. HPV16 E6 increased the internalization of activated receptor species, and the signaling adaptor protein GRB2 was shown to be critical for HPV16 E6 mediated enhanced EGFR internalization and mTORC1 activation. As a consequence of receptor protein kinase mediated mTORC1 activation, HPV16 E6 expression increased cellular migration of primary human epithelial cells. This study identifies a previously unappreciated mechanism by which HPV E6 proteins perturb host-signaling pathways presumably to sustain protein synthesis during the viral life cycle that may also contribute to cellular transforming activities of high-risk HPV E6 proteins.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Scheffner M, Huibregtse JM, Vierstra RD, Howley PM (1993) The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 75: 495–505. - PubMed
-
- Gardiol D, Kuhne C, Glaunsinger B, Lee SS, Javier R, et al. (1999) Oncogenic human papillomavirus E6 proteins target the discs large tumour suppressor for proteasome-mediated degradation. Oncogene 18: 5487–5496. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

