Antipurinergic therapy corrects the autism-like features in the poly(IC) mouse model
- PMID: 23516405
- PMCID: PMC3596371
- DOI: 10.1371/journal.pone.0057380
Antipurinergic therapy corrects the autism-like features in the poly(IC) mouse model
Abstract
Background: Autism spectrum disorders (ASDs) are caused by both genetic and environmental factors. Mitochondria act to connect genes and environment by regulating gene-encoded metabolic networks according to changes in the chemistry of the cell and its environment. Mitochondrial ATP and other metabolites are mitokines-signaling molecules made in mitochondria-that undergo regulated release from cells to communicate cellular health and danger to neighboring cells via purinergic signaling. The role of purinergic signaling has not yet been explored in autism spectrum disorders.
Objectives and methods: We used the maternal immune activation (MIA) mouse model of gestational poly(IC) exposure and treatment with the non-selective purinergic antagonist suramin to test the role of purinergic signaling in C57BL/6J mice.
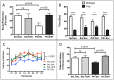
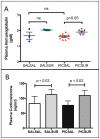
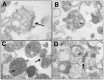
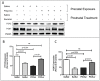
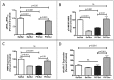
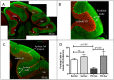
Results: We found that antipurinergic therapy (APT) corrected 16 multisystem abnormalities that defined the ASD-like phenotype in this model. These included correction of the core social deficits and sensorimotor coordination abnormalities, prevention of cerebellar Purkinje cell loss, correction of the ultrastructural synaptic dysmorphology, and correction of the hypothermia, metabolic, mitochondrial, P2Y2 and P2X7 purinergic receptor expression, and ERK1/2 and CAMKII signal transduction abnormalities.
Conclusions: Hyperpurinergia is a fundamental and treatable feature of the multisystem abnormalities in the poly(IC) mouse model of autism spectrum disorders. Antipurinergic therapy provides a new tool for refining current concepts of pathogenesis in autism and related spectrum disorders, and represents a fresh path forward for new drug development.
Conflict of interest statement
Figures




Comment in
-
Extracellular mitochondrial ATP, suramin, and autism?Clin Ther. 2013 Sep;35(9):1454-6. doi: 10.1016/j.clinthera.2013.07.419. Epub 2013 Aug 15. Clin Ther. 2013. PMID: 23954092 No abstract available.
References
-
- Buie T, Fuchs GJ III, Furuta GT, Kooros K, Levy J, et al. (2010) Recommendations for evaluation and treatment of common gastrointestinal problems in children with ASDs. Pediatrics 125 Suppl 1: S19–29. - PubMed
-
- Mulder EJ, Anderson GM, Kema IP, de Bildt A, van Lang ND, et al. (2004) Platelet serotonin levels in pervasive developmental disorders and mental retardation: diagnostic group differences, within-group distribution, and behavioral correlates. J Am Acad Child Adolesc Psychiatry 43: 491–499. - PubMed
-
- Palmieri L, Papaleo V, Porcelli V, Scarcia P, Gaita L, et al. (2010) Altered calcium homeostasis in autism-spectrum disorders: evidence from biochemical and genetic studies of the mitochondrial aspartate/glutamate carrier AGC1. Mol Psychiatry 15: 38–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

