Mapping the interactions of dengue virus NS1 protein with human liver proteins using a yeast two-hybrid system: identification of C1q as an interacting partner
- PMID: 23516407
- PMCID: PMC3597719
- DOI: 10.1371/journal.pone.0057514
Mapping the interactions of dengue virus NS1 protein with human liver proteins using a yeast two-hybrid system: identification of C1q as an interacting partner
Abstract
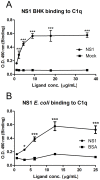
Dengue constitutes a global health concern. The clinical manifestation of this disease varies from mild febrile illness to severe hemorrhage and/or fatal hypovolemic shock. Flavivirus nonstructural protein 1 (NS1) is a secreted glycoprotein that is displayed on the surface of infected cells but is absent in viral particles. NS1 accumulates at high levels in the plasma of dengue virus (DENV)-infected patients, and previous reports highlight its involvement in immune evasion, dengue severity, liver dysfunction and pathogenesis. In the present study, we performed a yeast two-hybrid screen to search for DENV2 NS1-interacting partners using a human liver cDNA library. We identified fifty genes, including human complement component 1 (C1q), which was confirmed by coimmunoprecipitation, ELISA and immunofluorescence assays, revealing for the first time the direct binding of this protein to NS1. Furthermore, the majority of the identified genes encode proteins that are secreted into the plasma of patients, and most of these proteins are classified as acute-phase proteins (APPs), such as plasminogen, haptoglobin, hemopexin, α-2-HS-glycoprotein, retinol binding protein 4, transferrin, and C4. The results presented here confirm the direct interaction of DENV NS1 with a key protein of the complement system and suggest a role for this complement protein in the pathogenesis of DENV infection.
Conflict of interest statement
Figures



References
-
- Kyle JL, Harris E (2008) Global spread and persistence of dengue. Annu Rev Microbiol 62: 71–92. - PubMed
-
- Halstead SB (2007) Dengue. Lancet 370: 1644–1652. - PubMed
-
- Halstead SB (2003) Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res 60: 421–467. - PubMed
-
- Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S, et al. (2000) Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis 181: 2–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

