Anti-angiogenic and anti-metastatic activity of synthetic phosphoethanolamine
- PMID: 23516420
- PMCID: PMC3597720
- DOI: 10.1371/journal.pone.0057937
Anti-angiogenic and anti-metastatic activity of synthetic phosphoethanolamine
Abstract
Background: Renal cell carcinoma (RCC) is the most common type of kidney cancer, and represents the third most common urological malignancy. Despite the advent of targeted therapies for RCC and the improvement of the lifespan of patients, its cost-effectiveness restricted the therapeutic efficacy. In a recent report, we showed that synthetic phosphoethanolamine (Pho-s) has a broad antitumor activity on a variety of tumor cells and showed potent inhibitor effects on tumor progress in vivo.
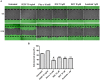
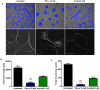
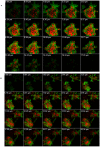
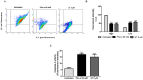
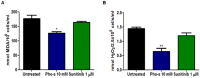
Methodology/principal findings: We show that murine renal carcinoma (Renca) is more sensitive to Pho-s when compared to normal immortalized rat proximal tubule cells (IRPTC) and human umbilical vein endothelial cells (HUVEC). In vitro anti-angiogenic activity assays show that Pho-s inhibits endothelial cell proliferation, migration and tube formation. In addition, Pho-s has anti-proliferative effects on HUVEC by inducing a cell cycle arrest at the G2/M phase. It causes a decrease in cyclin D1 mRNA, VEGFR1 gene transcription and VEGFR1 receptor expression. Pho-s also induces nuclear fragmentation and affects the organization of the cytoskeleton through the disruption of actin filaments. Additionally, Pho-s induces apoptosis through the mitochondrial pathway. The putative therapeutic potential of Pho-s was validated in a renal carcinoma model, on which our remarkable in vivo results show that Pho-s potentially inhibits lung metastasis in nude mice, with a superior efficacy when compared to Sunitinib.
Conclusions/significance: Taken together, our findings provide evidence that Pho-s is a compound that potently inhibits lung metastasis, suggesting that it is a promising novel candidate drug for future developments.
Conflict of interest statement
Figures


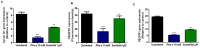


References
-
- SEER Star Database: Incidence SEER 9 Regs Public-Use, November 2002 Sub (1973–2000). (2003) Surveillance, Epidemiology, and End Results (SEER) Program. Bethesda: National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program, Cancer Statistics Branch.
-
- Garcia-Donas J, Esteban E, Leandro-García LJ, Castellano DE, del Alba AG, et al. (2011) Single nucleotide polymorphism associations with response and toxic effects in patients with advanced renal-cell carcinoma treated with first-line Sunitinib: a multicentre, observational, prospective study. Lancet Oncol 12: 1143–1150. - PubMed
-
- Ralph SJ, Rodríguez-Enríquez S, Neuzil J, Saavedra E, Moreno-Sánchez R (2010) The causes of cancer revisited: “mitochondrial malignancy” and ROS-induced oncogenic transformation-why mitochondria are targets for cancer therapy. Mol Aspects Med 31: 145–170. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

