Myostatin suppression of Akirin1 mediates glucocorticoid-induced satellite cell dysfunction
- PMID: 23516508
- PMCID: PMC3596298
- DOI: 10.1371/journal.pone.0058554
Myostatin suppression of Akirin1 mediates glucocorticoid-induced satellite cell dysfunction
Abstract
Glucocorticoids production is increased in many pathological conditions that are associated with muscle loss, but their role in causing muscle wasting is not fully understood. We have demonstrated a new mechanism of glucocorticoid-induced muscle atrophy: Dexamethasone (Dex) suppresses satellite cell function contributing to the development of muscle atrophy. Specifically, we found that Dex decreases satellite cell proliferation and differentiation in vitro and in vivo. The mechanism involved Dex-induced upregulation of myostatin and suppression of Akirin1, a promyogenic gene. When myostatin was inhibited in Dex-treated mice, Akirin1 expression increased as did satellite cell activity, muscle regeneration and muscle growth. In addition, silencing myostatin in myoblasts or satellite cells prevented Dex from suppressing Akirin1 expression and cellular proliferation and differentiation. Finally, overexpression of Akirin1 in myoblasts increased their expression of MyoD and myogenin and improved cellular proliferation and differentiation, theses improvements were no longer suppressed by Dex. We conclude that glucocorticoids stimulate myostatin which inhibits Akirin1 expression and the reparative functions of satellite cells. These responses attribute to muscle atrophy. Thus, inhibition of myostatin or increasing Akirin1 expression could lead to therapeutic strategies for improving satellite cell activation and enhancing muscle growth in diseases associated with increased glucocorticoid production.
Conflict of interest statement
Figures

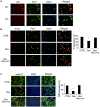
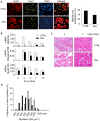
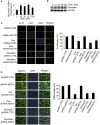
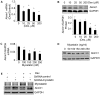
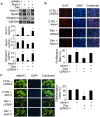
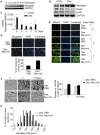
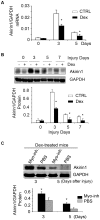
References
-
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, et al. (2000) Pax7 is required for the specification of myogenic satellite cells. Cell 102: 777–786. - PubMed
-
- Mitchell PO, Pavlath GK (2004) Skeletal muscle atrophy leads to loss and dysfunction of muscle precursor cells. Am J Physiol Cell Physiol 287: C1753–C1762. - PubMed
-
- Jejurikar SS, Kuzon WM Jr (2003) Satellite cell depletion in degenerative skeletal muscle. Apoptosis 8: 573–578. - PubMed
-
- Hasselgren PO (1999) Glucocorticoids and muscle catabolism. Curr Opin Clin Nutr Metab Care 2: 201–205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

