Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans
- PMID: 23516617
- PMCID: PMC3597605
- DOI: 10.1371/journal.pone.0059260
Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans
Abstract
Background: The human intestinal microbiota is a crucial factor in the pathogenesis of various diseases, such as metabolic syndrome or inflammatory bowel disease (IBD). Yet, knowledge about the role of environmental factors such as smoking (which is known to influence theses aforementioned disease states) on the complex microbial composition is sparse. We aimed to investigate the role of smoking cessation on intestinal microbial composition in 10 healthy smoking subjects undergoing controlled smoking cessation.
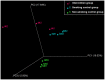
Methods: During the observational period of 9 weeks repetitive stool samples were collected. Based on abundance of 16S rRNA genes bacterial composition was analysed and compared to 10 control subjects (5 continuing smokers and 5 non-smokers) by means of Terminal Restriction Fragment Length Polymorphism analysis and high-throughput sequencing.
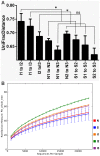
Results: Profound shifts in the microbial composition after smoking cessation were observed with an increase of Firmicutes and Actinobacteria and a lower proportion of Bacteroidetes and Proteobacteria on the phylum level. In addition, after smoking cessation there was an increase in microbial diversity.
Conclusions: These results indicate that smoking is an environmental factor modulating the composition of human gut microbiota. The observed changes after smoking cessation revealed to be similar to the previously reported differences in obese compared to lean humans and mice respectively, suggesting a potential pathogenetic link between weight gain and smoking cessation. In addition they give rise to a potential association of smoking status and the course of IBD.
Conflict of interest statement
Figures

References
-
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL (2005) An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 122 (1): 107–118. - PubMed
-
- Sonnenburg JL, Xu J, Leip DD, Chen C, Westover BP, et al. (2005) Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 307 (5717): 1955–1959. - PubMed
-
- Seksik P, Nion-Larmurier I, Sokol H, Beaugerie L, Cosnes J (2009) Effects of light smoking consumption on the clinical course of Crohn's disease. Inflamm. Bowel Dis. 15 (5): 734–741. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

