Proof of concept of the ability of the kinect to quantify upper extremity function in dystrophinopathy
- PMID: 23516667
- PMCID: PMC3600356
- DOI: 10.1371/currents.md.9ab5d872bbb944c6035c9f9bfd314ee2
Proof of concept of the ability of the kinect to quantify upper extremity function in dystrophinopathy
Abstract
Introduction: Individuals with dystrophinopathy lose upper extremity strength in proximal muscles followed by those more distal. Current upper extremity evaluation tools fail to fully capture changes in upper extremity strength and function across the disease spectrum as they tend to focus solely on distal ability. The Kinect by Microsoft is a gaming interface that can gather positional information about an individual's upper extremity movement which can be used to determine functional reaching volume, velocity of movement, and rate of fatigue while playing an engaging video game. The purpose of this study was to determine the feasibility of using the Kinect platform to assess upper extremity function in individuals with dystrophinopathy across the spectrum of abilities.
Methods: Investigators developed a proof-of-concept device, ACTIVE (Abilities Captured Through Interactive Video Evaluation), to measure functional reaching volume, movement velocity, and rate of fatigue. Five subjects with dystrophinopathy and 5 normal controls were tested using ACTIVE during one testing session. A single subject with dystrophinopathy was simultaneously tested with ACTIVE and a marker-based motion analysis system to establish preliminary validity of measurements.
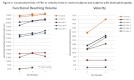
Results: ACTIVE proof-of-concept ranked the upper extremity abilities of subjects with dystrophinopathy by Brooke score, and also differentiated them from performance of normal controls for the functional reaching volume and velocity tests. Preliminary test-retest reliability of the ACTIVE for 2 sequential trials was excellent for functional reaching volume (ICC=0.986, p<0.001) and velocity trials (ICC=0.963, p<0.001).
Discussion: The data from our pilot study with ACTIVE proof-of-concept demonstrates that newly available gaming technology has potential to be used to create a low-cost, widely-accessible and functional upper extremity outcome measure for use with children and adults with dystrophinopathy.
References
-
- Mendell JR, Province MA, Moxley RT 3rd, Griggs RC, Brooke MH, Fenichel GM, Miller JP, Kaiser KK, King W, Robison J. Clinical investigation of Duchenne muscular dystrophy. A methodology for therapeutic trials based on natural history controls. Arch Neurol. 1987 Aug;44(8):808-11. PubMed PMID:3115236. - PubMed
-
- Brooke MH, Fenichel GM, Griggs RC, Mendell JR, Moxley R, Miller JP, Province MA. Clinical investigation in Duchenne dystrophy: 2. Determination of the "power" of therapeutic trials based on the natural history. Muscle Nerve. 1983 Feb;6(2):91-103. PubMed PMID:6343858. - PubMed
-
- Baiardini I, Minetti C, Bonifacino S, Porcu A, Klersy C, Petralia P, Balestracci S, Tarchino F, Parodi S, Canonica GW, Braido F. Quality of life in Duchenne muscular dystrophy: the subjective impact on children and parents. J Child Neurol. 2011 Jun;26(6):707-13. PubMed PMID:21482750. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources