Immunohistochemical and ultrastructural features of congenital melanocytic naevus cells support a stem-cell phenotype
- PMID: 23517330
- PMCID: PMC3838625
- DOI: 10.1111/bjd.12323
Immunohistochemical and ultrastructural features of congenital melanocytic naevus cells support a stem-cell phenotype
Abstract
Background: Multiple congenital melanocytic naevi (CMN) in one individual are caused by somatic mosaicism for NRAS mutations; however, the lineage of the mutated cells remains uncertain.
Objectives: To test the hypothesis that CMN may be derived from cutaneous stem cells.
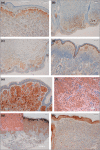
Methods: Sixty-six CMN samples from 44 patients were stained for immunohistochemical (IHC) markers of melanocytic differentiation (TYR, TRP1, TRP2, LEF1, MITF, cKit), pluripotency (nestin, fascin, CD133, CD20, CD34), monocyte/macrophage lineage (CD68, CD163, CD14), proliferation (Ki67) and MTOR/Wnt-signalling pathway activation (pS6, β-catenin). Semiquantitative scoring compared samples with naevus cell nesting (group 1) with those with only diffuse dermal infiltration (group 2). Transmission electron microscopy (TEM) was performed on 10 samples.
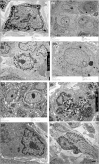
Results: A normal melanocyte population was seen overlying many dermal CMN. Group 1 samples were significantly more likely to express melanocytic differentiation markers than group 2, and expression decreased significantly with depth. Expression of these markers was correlated with each other, and with nestin and fascin. CD20 staining was positive in a substantial proportion and was stronger superficially. Expression of β-catenin and pS6 was almost universal. Some samples expressed monocyte/macrophage markers. TEM revealed variable naevus cell morphology, striking macromelanosomes, double cilia and microvilli.
Conclusions: Congenital melanocytic naevi development frequently coexists with normal overlying melanocyte development, leading us to hypothesize that in these cases CMN are likely to develop from a cell present in the skin independent of, or remaining after, normal melanocytic migration. IHC and TEM findings are compatible with CMN cells being of cutaneous stem-cell origin, capable of some degree of melanocytic differentiation superficially.
© 2013 The Authors BJD © 2013 British Association of Dermatologists.
Figures



References
-
- Barnhill RL, Piepkorn MW, Busam KJ. Pathology of Melanocytic Nevi and Malignant Melanoma. New York, NY: Springer; 2004.
-
- Clemmensen OJ, Kroon S. The histology of ‘congenital features’ in early acquired melanocytic nevi. J Am Acad Dermatol. 1988;19:742–6. - PubMed
-
- Misago N. The relationship between melanocytes and peripheral nerve sheath cells (Part I): melanocytic nevus (excluding so-called ‘blue nevus’) with peripheral nerve sheath differentiation. Am J Dermatopathol. 2000;22:217–29. - PubMed
-
- McCann E, Fryer AE, Kokai G. Congenital melanocytic naevus with associated neurofibroma and schwannoma-like change. Clin Dysmorphol. 2005;14:159–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

