Reactivation of latent HIV by histone deacetylase inhibitors
- PMID: 23517573
- PMCID: PMC3685471
- DOI: 10.1016/j.tim.2013.02.005
Reactivation of latent HIV by histone deacetylase inhibitors
Abstract
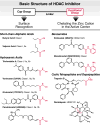
Latent HIV persists in CD4(+) T cells in infected patients under antiretroviral therapy (ART). Latency is associated with transcriptional silencing of the integrated provirus and driven, at least in part, by histone deacetylases (HDACs), a family of chromatin-associated proteins that regulate histone acetylation and the accessibility of DNA to transcription factors. Remarkably, inhibition of HDACs is sufficient to reactivate a fraction of latent HIV in a variety of experimental systems. This basic observation led to the shock and kill idea that forcing the transcriptional activation of HIV might lead to virus expression, to virus- or host-induced cell death of the reactivated cells, and to the eradication of the pool of latently infected cells. Such intervention might possibly lead to a cure for HIV-infected patients. Here, we review the basic biology of HDACs and their inhibitors, the role of HDACs in HIV latency, and recent efforts to use HDAC inhibitors to reactivate latent HIV in vitro and in vivo.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

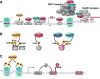
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

