The role of RhoA and cytoskeleton in myofibroblast transformation in hyperoxic lung fibrosis
- PMID: 23517783
- PMCID: PMC3849210
- DOI: 10.1016/j.freeradbiomed.2013.03.012
The role of RhoA and cytoskeleton in myofibroblast transformation in hyperoxic lung fibrosis
Abstract
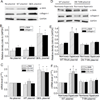
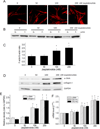
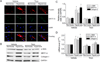
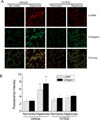
Myofibroblast transformation is a key process in the pathogenesis of lung fibrosis. We have previously reported that hyperoxia induces RhoA activation in HFL-1 lung fibroblasts and RhoA mediates collagen synthesis in hyperoxic lung fibrosis. In this study, we investigated the role of RhoA and actin cytoskeleton in hyperoxia-induced myofibroblast transformation. Exposure of HFL-1 lung fibroblasts to hyperoxia stimulated actin filament formation, shift of G-actin to F-actin, nuclear colocalization of myocardin-related transcription factor-A (MRTF-A), recruitment of MRTF-A to the α-smooth muscle actin (α-SMA) gene promoter, myofibroblast transformation, and collagen-I synthesis. Inhibition of RhoA by C3 transferase CT-04 or dominant-negative RhoA mutant T19N, and inhibition of ROCK by Y27632, prevented myofibroblast transformation and collagen-I synthesis. Moreover, inhibition of RhoA by CT-04 prevented hyperoxia-induced actin filament formation, shift of G-actin to F-actin, and nuclear colocalization of MRTF-A. In addition, disrupting actin filaments with cytochalasin D or scavenging reactive oxygen species (ROS) with tiron attenuated actin filament formation, nuclear colocalization of MRTF-A, myofibroblast transformation, and collagen-I synthesis. Furthermore, overexpression of constitutively active RhoA mutant Q63L or stabilization of actin filaments recapitulated the effects of hyperoxia on the actin cytoskeleton and nuclear colocalization of MRTF-A, myofibroblast transformation, and collagen-I synthesis. Interestingly, knocking down MRTF-A prevented hyperoxia-induced increase in the recruitment of MRTF-A to the serum response factor transcriptional complex on the α-SMA gene promoter, myofibroblast transformation, and collagen-I synthesis. Finally, Y27632 and tiron attenuated hyperoxia-induced increases in α-SMA and collagen-I in mouse lungs. Together, these results indicate that the actin cytoskeletal reorganization due to the ROS/RhoA-ROCK pathway mediates myofibroblast transformation and collagen synthesis in lung fibrosis of oxygen toxicity. MRTF-A contributes to the regulatory effect of the actin cytoskeleton on myofibroblast transformation during hyperoxia.
Keywords: Collagen; Fibroblasts; Free radicals; Lung; MRTF-A; Oxygen toxicity; Reactive oxygen species.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures









References
-
- Meyrick B. Pathology of the adult respiratory distress syndrome. Crit Care Clin. 1986;2:405–428. - PubMed
-
- Davis WB, Rennard SI, Bitterman PB, Crystal RG. Pulmonary oxygen toxicity. Early reversible changes in human alveolar structures induced by hyperoxia. N. Engl. J. Med. 1983;309:878–883. - PubMed
-
- Riley DJ, Kerr JS, Yu SY. Effect of proline analogs on oxygen toxicity-induced pulmonary fibrosis in the rat. Toxicol. Appl. Pharmacol. 1984;75:554–560. - PubMed
-
- Weinberger B, Laskin DL, Heck DE, Laskin JD. Oxygen toxicity in premature infants. Toxicol. Appl. Pharmacol. 2002;181:60–67. - PubMed
-
- Chen N, Li JJ, Xue XD. [Effect of losartan on lung fibrosis in neonatal rats with hyperoxia-induced chronic lung disease] Zhongguo Dang. Dai Er. Ke. Za Zhi. 2007;9:591–594. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

