Combining a dopamine agonist and selective serotonin reuptake inhibitor for the treatment of depression: a double-blind, randomized pilot study
- PMID: 23517885
- PMCID: PMC3672377
- DOI: 10.1016/j.jad.2013.02.003
Combining a dopamine agonist and selective serotonin reuptake inhibitor for the treatment of depression: a double-blind, randomized pilot study
Abstract
Background: Antidepressants that act on two or more amine neurotransmitters may confer higher remission rates when first-line agents affecting a single neurotransmitter have failed. Pramipexole, a dopamine agonist, has antidepressant effects in patients with major depressive disorder (MDD). This pilot study examined the efficacy and safety of combination therapy with pramipexole and the selective serotonin reuptake inhibitor (SSRI) escitalopram in MDD.
Methods: In this double-blind, controlled, pilot study, 39 patients with DSM-IV MDD who had failed to respond to a standard antidepressant treatment trial were randomized to receive pramipexole (n=13), escitalopram (n=13), or their combination (n=13) for six weeks. Pramipexole was started at 0.375 mg/day and titrated weekly up to 2.25 mg/day; escitalopram dosage remained at 10 mg/day. The primary outcome measure was the Montgomery-Asberg Depression Rating Scale (MADRS).
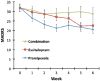
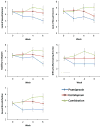
Results: Subjects receiving pramipexole monotherapy had significantly lower MADRS scores than the combination group (p=0.01); no other primary drug comparisons were significant. The combination group had a substantially higher dropout rate than the escitalopram and pramipexole groups (69%, 15%, 15%, respectively). Only 15% of patients in the combination group tolerated regularly scheduled increases of pramipexole throughout the study, compared with 46% of patients in the pramipexole group.
Limitations: Group size was small and the treatment phase lasted for only six weeks.
Conclusions: The combination of an SSRI and a dopamine agonist was not more effective than either agent alone, nor did it produce a more rapid onset of antidepressant action. Combination therapy with escitalopram and pramipexole may not be well-tolerated.
Published by Elsevier B.V.
Conflict of interest statement
All other authors report no conflict of interest, financial or otherwise.
Figures
References
-
- American Psychiatric Association. Practice Guideline for the Treatment of Patients with Major Depressive Disorder. 3. American Psychiatric Association; Arlington, VA: 2010. - PubMed
-
- Antonini A, Barone P, Ceravolo R, Fabbrini G, Tinazzi M, Abbruzzese G. Role of pramipexole in the management of Parkinson’s disease. CNS Drugs. 2010;24:829–841. - PubMed
-
- Aurora RN, Kristo DA, Bista SR, Rowley JA, Zak RS, Casey KR, Lamm CI, Tracy SL, Rosenberg RS. The treatment of restless legs syndrome and periodic limb movement disorder in adults-an update for 2012: practice parameters with an evidence-based systematic review and meta-analyses: an american academy of sleep medicine clinical practice guideline. Sleep. 2012;35:1039–1062. - PMC - PubMed
-
- Baldomero EB, Ubago JG, Cercos CL, Ruiloba JV, Calvo CG, Lopez RP. Venlafaxine extended release versus conventional antidepressants in the remission of depressive disorders after previous antidepressant failure: ARGOS study. Depression and Anxiety. 2005;22:68–76. - PubMed
-
- Baldwin DS, Cooper JA, Huusom AK, Hindmarch I. A double-blind, randomized, parallel-group, flexible-dose study to evaluate the tolerability, efficacy and effects of treatment discontinuation with escitalopram and paroxetine in patients with major depressive disorder. International Clinical Psychopharmacology. 2006;21:159–169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

