Titanium particle-challenged osteoblasts promote osteoclastogenesis and osteolysis in a murine model of periprosthestic osteolysis
- PMID: 23518478
- PMCID: PMC3686639
- DOI: 10.1016/j.actbio.2013.03.010
Titanium particle-challenged osteoblasts promote osteoclastogenesis and osteolysis in a murine model of periprosthestic osteolysis
Abstract
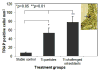
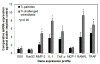
The current study investigates the interactive behavior of titanium alloy particle-challenged osteoblastic bone marrow stromal cells (BMSCs) and macrophage lineage cells in a murine knee-prosthesis failure model. BMSCs were isolated from male BALB/c mice femurs and induced in osteogenic medium. At 24h after isolation, BMSCs in complete induction medium were challenged with 1, 3 or 5mgml(-1) titanium particles for 7days. Culture media were collected at 2, 4 and 6days and cells were harvested at 7days for alkaline phosphatase (ALP) assay/stains. Cell proliferation in the presence of Ti particles was periodically evaluated by MTT assay. Mice implanted with titanium-pin tibial implants were given an intra-articular injection of 50μl medium containing 5×10(5) Ti particles-challenged bone-marrow-derived osteoblastic cells, followed by a repeat injection at 2weeks post-operation. Control mice with titanium-pin implants received a naïve osteoblastic cell transfusion. After sacrifice at 4weeks, the implanted knee joint of each group was collected for biomechanical pin-pullout testing, histological evaluation and reverse transcriptase polymerase chain reaction analysis of mRNA extracted from the joint tissues. Ti particles significantly stimulated the proliferation of BMSC-derived osteoblastic cells at both high and low particle concentrations (p<0.05), with no marked differences between the particle doses. ALP expression was diminished following Ti particle interactions, especially in the high-dose particle group (p<0.05). In addition, the culture media collected from short-term challenged (48h) osteoblasts significantly increased the numbers of TRAP+ cells when added to mouse peripheral blood monocytes cultures, in comparison with the monocytes cells receiving naïve osteoblasts media (p<0.05). Intra-articular introduction of the osteoblastic cells to the mouse pin-implant failure model resulted in reduced implant interfacial shear strength and thicker peri-implant soft-tissue formation, suggesting that titanium particles-challenged osteoblasts contributed to periprosthetic osteolysis. Comparison of the gene expression profiles among the peri-implant tissue samples following osteoblast injection did not find significant difference in RunX2 or Osterix/Sp7 between the groups. However, MMP-2, IL-1, TNF-α, RANKL, and TRAP gene expressions were elevated in the challenged-osteoblast group (p<0.05). In conclusion, titanium alloy particles were shown to interfere with the growth, maturation, and functions of the bone marrow osteoblast progenitor cells. Particle-challenged osteoblasts appear to express mediators that regulate osteoclastogenesis and peri-prosthetic osteolysis.
Copyright © 2013 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
All authors report no conflicts of interest and are responsible for the content of the manuscript.
Figures





Similar articles
-
Particulate and ion forms of cobalt-chromium challenged preosteoblasts promote osteoclastogenesis and osteolysis in a murine model of prosthesis failure.J Biomed Mater Res A. 2019 Jan;107(1):187-194. doi: 10.1002/jbm.a.36553. Epub 2018 Oct 25. J Biomed Mater Res A. 2019. PMID: 30358096 Free PMC article.
-
Curculigoside Protects against Titanium Particle-Induced Osteolysis through the Enhancement of Osteoblast Differentiation and Reduction of Osteoclast Formation.J Immunol Res. 2021 Jul 4;2021:5707242. doi: 10.1155/2021/5707242. eCollection 2021. J Immunol Res. 2021. PMID: 34285923 Free PMC article.
-
Effects of Ti, PMMA, UHMWPE, and Co-Cr wear particles on differentiation and functions of bone marrow stromal cells.J Biomed Mater Res A. 2013 Oct;101(10):2817-25. doi: 10.1002/jbm.a.34595. Epub 2013 Mar 5. J Biomed Mater Res A. 2013. PMID: 24039045 Free PMC article.
-
Wear and osteolysis in total joint replacements.Acta Orthop Scand Suppl. 1998 Feb;278:1-16. Acta Orthop Scand Suppl. 1998. PMID: 9524528 Review.
-
Osteolysis around total knee arthroplasty: a review of pathogenetic mechanisms.Acta Biomater. 2013 Sep;9(9):8046-58. doi: 10.1016/j.actbio.2013.05.005. Epub 2013 May 10. Acta Biomater. 2013. PMID: 23669623 Free PMC article. Review.
Cited by
-
Effect of Implant Surface Roughness and Macro- and Micro-Structural Composition on Wear and Metal Particles Released.Materials (Basel). 2021 Nov 11;14(22):6800. doi: 10.3390/ma14226800. Materials (Basel). 2021. PMID: 34832201 Free PMC article.
-
Mettl3‑mediated m6A RNA methylation regulates osteolysis induced by titanium particles.Mol Med Rep. 2024 Mar;29(3):36. doi: 10.3892/mmr.2024.13160. Epub 2024 Jan 12. Mol Med Rep. 2024. PMID: 38214327 Free PMC article.
-
Magnoflorine Suppresses MAPK and NF-κB Signaling to Prevent Inflammatory Osteolysis Induced by Titanium Particles In Vivo and Osteoclastogenesis via RANKL In Vitro.Front Pharmacol. 2020 Apr 2;11:389. doi: 10.3389/fphar.2020.00389. eCollection 2020. Front Pharmacol. 2020. PMID: 32300300 Free PMC article.
-
Type-2 cannabinoid receptor regulates proliferation, apoptosis, differentiation, and OPG/RANKL ratio of MC3T3-E1 cells exposed to Titanium particles.Mol Cell Biochem. 2015 Jan;399(1-2):131-41. doi: 10.1007/s11010-014-2240-y. Epub 2014 Oct 8. Mol Cell Biochem. 2015. PMID: 25292314
-
Particulate and ion forms of cobalt-chromium challenged preosteoblasts promote osteoclastogenesis and osteolysis in a murine model of prosthesis failure.J Biomed Mater Res A. 2019 Jan;107(1):187-194. doi: 10.1002/jbm.a.36553. Epub 2018 Oct 25. J Biomed Mater Res A. 2019. PMID: 30358096 Free PMC article.
References
-
- Bozic KJ, Kurtz SM, Lau E, Ong K, Vail TP, Berry DJ. The epidemiology of revision total hip arthroplasty in the United States. J of Bone Joint Surg [AM] 2009;91:128–33. - PubMed
-
- Kadoya Y, Revell PA, Kobayashi A, al-Saffar N, Scott G, Freeman MA. Wear particulate species and bone loss in failed total joint arthroplasties. Clinical orthopaedics and related research. 1997:118–29. - PubMed
-
- Kim KJ, Chiba J, Rubash HE. In vivo and in vitro analysis of membranes from hip prostheses inserted without cement. J Bone Joint Surg Am. 1994;76:172–80. - PubMed
-
- Maloney WJ, Smith RL, Schmalzried TP, Chiba J, Huene D, Rubash H. Isolation and characterization of wear particles generated in patients who have had failure of a hip arthroplasty without cement. J Bone Joint Surg Am. 1995;77:1301–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

