A genetic study of Wilson's disease in the United Kingdom
- PMID: 23518715
- PMCID: PMC3634195
- DOI: 10.1093/brain/awt035
A genetic study of Wilson's disease in the United Kingdom
Abstract
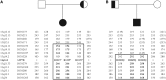
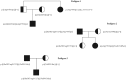
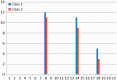
Previous studies have failed to identify mutations in the Wilson's disease gene ATP7B in a significant number of clinically diagnosed cases. This has led to concerns about genetic heterogeneity for this condition but also suggested the presence of unusual mutational mechanisms. We now present our findings in 181 patients from the United Kingdom with clinically and biochemically confirmed Wilson's disease. A total of 116 different ATP7B mutations were detected, 32 of which are novel. The overall mutation detection frequency was 98%. The likelihood of mutations in genes other than ATP7B causing a Wilson's disease phenotype is therefore very low. We report the first cases with Wilson's disease due to segmental uniparental isodisomy as well as three patients with three ATP7B mutations and three families with Wilson's disease in two consecutive generations. We determined the genetic prevalence of Wilson's disease in the United Kingdom by sequencing the entire coding region and adjacent splice sites of ATP7B in 1000 control subjects. The frequency of all single nucleotide variants with in silico evidence of pathogenicity (Class 1 variant) was 0.056 or 0.040 if only those single nucleotide variants that had previously been reported as mutations in patients with Wilson's disease were included in the analysis (Class 2 variant). The frequency of heterozygote, putative or definite disease-associated ATP7B mutations was therefore considerably higher than the previously reported occurrence of 1:90 (or 0.011) for heterozygote ATP7B mutation carriers in the general population (P < 2.2 × 10(-16) for Class 1 variants or P < 5 × 10(-11) for Class 2 variants only). Subsequent exclusion of four Class 2 variants without additional in silico evidence of pathogenicity led to a further reduction of the mutation frequency to 0.024. Using this most conservative approach, the calculated frequency of individuals predicted to carry two mutant pathogenic ATP7B alleles is 1:7026 and thus still considerably higher than the typically reported prevalence of Wilson's disease of 1:30 000 (P = 0.00093). Our study provides strong evidence for monogenic inheritance of Wilson's disease. It also has major implications for ATP7B analysis in clinical practice, namely the need to consider unusual genetic mechanisms such as uniparental disomy or the possible presence of three ATP7B mutations. The marked discrepancy between the genetic prevalence and the number of clinically diagnosed cases of Wilson's disease may be due to both reduced penetrance of ATP7B mutations and failure to diagnose patients with this eminently treatable disorder.
Figures


References
-
- Ala A, Borjigin J, Rochwarger A, Schilsky M. Wilson disease in septuagenarian siblings: Raising the bar for diagnosis. Hepatology. 2005;41:668–70. - PubMed
-
- Ala A, Walker AP, Ashkan K, Dooley JS, Schilsky ML. Wilson's disease. Lancet. 2007;369:397–408. - PubMed
-
- Bachmann H, Lossner J, Gruss B, Ruchholtz U. [The epidemiology of Wilson's disease in the German Democratic Republic and current problems from the viewpoint of population genetics] Psychiatr Neurol Med Psychol (Leipz) 1979;31:393–400. - PubMed
-
- Beyersdorff A, Findeisen A. Morbus Wilson: Case report of a two-year-old child as first manifestation. Scand J Gastroenterol. 2006;41:496–7. - PubMed
-
- Cullen LM, Prat L, Cox DW. Genetic variation in the promoter and 5' UTR of the copper transporter, ATP7B, in patients with Wilson disease. Clin Genet. 2003;64:429–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

