An essential role for insulin and IGF1 receptors in regulating sertoli cell proliferation, testis size, and FSH action in mice
- PMID: 23518924
- PMCID: PMC5416760
- DOI: 10.1210/me.2012-1258
An essential role for insulin and IGF1 receptors in regulating sertoli cell proliferation, testis size, and FSH action in mice
Abstract
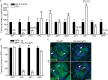
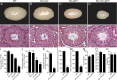
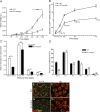
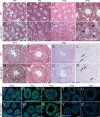
Testis size and sperm production are directly correlated to the total number of adult Sertoli cells (SCs). Although the establishment of an adequate number of SCs is crucial for future male fertility, the identification and characterization of the factors regulating SC survival, proliferation, and maturation remain incomplete. To investigate whether the IGF system is required for germ cell (GC) and SC development and function, we inactivated the insulin receptor (Insr), the IGF1 receptor (Igf1r), or both receptors specifically in the GC lineage or in SCs. Whereas ablation of insulin/IGF signaling appears dispensable for GCs and spermatogenesis, adult testes of mice lacking both Insr and Igf1r in SCs (SC-Insr;Igf1r) displayed a 75% reduction in testis size and daily sperm production as a result of a reduced proliferation rate of immature SCs during the late fetal and early neonatal testicular period. In addition, in vivo analyses revealed that FSH requires the insulin/IGF signaling pathway to mediate its proliferative effects on immature SCs. Collectively, these results emphasize the essential role played by growth factors of the insulin family in regulating the final number of SCs, testis size, and daily sperm output. They also indicate that the insulin/IGF signaling pathway is required for FSH-mediated SC proliferation.
Figures

References
-
- Jégou B. The Sertoli cell. Baillieres Clin. Endocrinol. Metab. 1992;6:273–311. - PubMed
-
- Mruk DD , Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806. - PubMed
-
- Orth JM , Gunsalus GL , Lamperti AA. Evidence from Sertoli cell-depleted rats indicates that spermatid number in adults depends on numbers of Sertoli cells produced during perinatal development. Endocrinology. 1988;122:787–794. - PubMed
-
- Baker PJ , O'Shaughnessy PJ. Role of gonadotrophins in regulating numbers of Leydig and Sertoli cells during fetal and postnatal development in mice. Reproduction. 2001;122:227–234. - PubMed
-
- Vergouwen RP , Jacobs SG , Huiskamp R , Davids JA , de Rooij DG. Proliferative activity of gonocytes, Sertoli cells and interstitial cells during testicular development in mice. J Reprod Fertil. 1991;93:233–243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

