Dicer deficiency reveals microRNAs predicted to control gene expression in the developing adrenal cortex
- PMID: 23518926
- PMCID: PMC3634112
- DOI: 10.1210/me.2012-1331
Dicer deficiency reveals microRNAs predicted to control gene expression in the developing adrenal cortex
Abstract
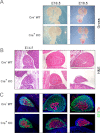
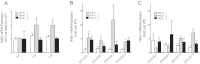
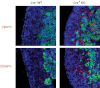
MicroRNAs (miRNAs) are small, endogenous, non-protein-coding RNAs that are an important means of posttranscriptional gene regulation. Deletion of Dicer, a key miRNA processing enzyme, is embryonic lethal in mice, and tissue-specific Dicer deletion results in developmental defects. Using a conditional knockout model, we generated mice lacking Dicer in the adrenal cortex. These Dicer-knockout (KO) mice exhibited perinatal mortality and failure of the adrenal cortex during late gestation between embryonic day 16.5 (E16.5) and E18.5. Further study of Dicer-KO adrenals demonstrated a significant loss of steroidogenic factor 1-expressing cortical cells that was histologically evident as early as E16.5 coincident with an increase in p21 and cleaved-caspase 3 staining in the cortex. However, peripheral cortical proliferation persisted in KO adrenals as assessed by staining of proliferating cell nuclear antigen. To further characterize the embryonic adrenals from Dicer-KO mice, we performed microarray analyses for both gene and miRNA expression on purified RNA isolated from control and KO adrenals of E15.5 and E16.5 embryos. Consistent with the absence of Dicer and the associated loss of miRNA-mediated mRNA degradation, we observed an up-regulation of a small subset of adrenal transcripts in Dicer-KO mice, most notably the transcripts coded by the genes Nr6a1 and Acvr1c. Indeed, several miRNAs, including let-7, miR-34c, and miR-21, that are predicted to target these genes for degradation, were also markedly down-regulated in Dicer-KO adrenals. Together these data suggest a role for miRNA-mediated regulation of a subset of genes that are essential for normal adrenal growth and homeostasis.
Figures




References
-
- Hammer GD, Parker KL, Schimmer BP. Minireview: transcriptional regulation of adrenocortical development. Endocrinology. 2005;146:1018–1024 - PubMed
-
- Lalli E. Adrenal cortex ontogenesis. Best Pract Res Clin Endocrinol Metab. 2010;24:853–864 - PubMed
-
- Parker KL, Schimmer BP. The roles of the nuclear receptor steroidogenic factor 1 in endocrine differentiation and development. Trends Endocrinol Metab. 1996;7:203–207 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

