Multifunctional T-cell analyses to study response and progression in adoptive cell transfer immunotherapy
- PMID: 23519018
- PMCID: PMC3716460
- DOI: 10.1158/2159-8290.CD-12-0383
Multifunctional T-cell analyses to study response and progression in adoptive cell transfer immunotherapy
Abstract
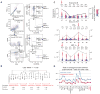
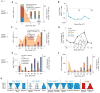
Adoptive cell transfer (ACT) of genetically engineered T cells expressing cancer-specific T-cell receptors (TCR) is a promising cancer treatment. Here, we investigate the in vivo functional activity and dynamics of the transferred cells by analyzing samples from 3 representative patients with melanoma enrolled in a clinical trial of ACT with TCR transgenic T cells targeted against the melanosomal antigen MART-1. The analyses included evaluating 19 secreted proteins from individual cells from phenotypically defined T-cell subpopulations, as well as the enumeration of T cells with TCR antigen specificity for 36 melanoma antigens. These analyses revealed the coordinated functional dynamics of the adoptively transferred, as well as endogenous, T cells, and the importance of highly functional T cells in dominating the antitumor immune response. This study highlights the need to develop approaches to maintaining antitumor T-cell functionality with the aim of increasing the long-term efficacy of TCR-engineered ACT immunotherapy.
Significance: A longitudinal functional study of adoptively transferred TCR–engineered lymphocytes yielded revealing snapshots for understanding the changes of antitumor responses over time in ACT immunotherapy of patients with advanced melanoma.
Trial registration: ClinicalTrials.gov NCT00910650.
©2013 AACR.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures


Comment in
-
Hit parade for adoptive cell transfer therapy: the best T cells for superior clinical responses.Cancer Discov. 2013 Apr;3(4):379-81. doi: 10.1158/2159-8290.CD-13-0064. Cancer Discov. 2013. PMID: 23580281
References
-
- Ribas A, Camacho LH, Lopez-Berestein G, Pavlov D, Bulanhagui CA, Millham R, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte–associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 2005;23:8968–77. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

