Annexin A2 heterotetramer: structure and function
- PMID: 23519104
- PMCID: PMC3634455
- DOI: 10.3390/ijms14036259
Annexin A2 heterotetramer: structure and function
Abstract
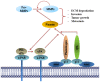
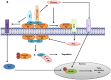
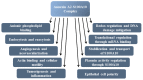
Annexin A2 is a pleiotropic calcium- and anionic phospholipid-binding protein that exists as a monomer and as a heterotetrameric complex with the plasminogen receptor protein, S100A10. Annexin A2 has been proposed to play a key role in many processes including exocytosis, endocytosis, membrane organization, ion channel conductance, and also to link F-actin cytoskeleton to the plasma membrane. Despite an impressive list of potential binding partners and regulatory activities, it was somewhat unexpected that the annexin A2-null mouse should show a relatively benign phenotype. Studies with the annexin A2-null mouse have suggested important functions for annexin A2 and the heterotetramer in fibrinolysis, in the regulation of the LDL receptor and in cellular redox regulation. However, the demonstration that depletion of annexin A2 causes the depletion of several other proteins including S100A10, fascin and affects the expression of at least sixty-one genes has confounded the reports of its function. In this review we will discuss the annexin A2 structure and function and its proposed physiological and pathological roles.
Figures



Similar articles
-
Phospholipid-associated annexin A2-S100A10 heterotetramer and its subunits: characterization of the interaction with tissue plasminogen activator, plasminogen, and plasmin.J Biol Chem. 2003 Jul 11;278(28):25577-84. doi: 10.1074/jbc.M301017200. Epub 2003 Apr 30. J Biol Chem. 2003. PMID: 12730231
-
S100A10, annexin A2, and annexin a2 heterotetramer as candidate plasminogen receptors.Front Biosci. 2005 Jan 1;10:300-25. doi: 10.2741/1529. Print 2005 Jan 1. Front Biosci. 2005. PMID: 15574370 Review.
-
The p11/S100A10 light chain of annexin A2 is dispensable for annexin A2 association to endosomes and functions in endosomal transport.PLoS One. 2007 Oct 31;2(10):e1118. doi: 10.1371/journal.pone.0001118. PLoS One. 2007. PMID: 17971878 Free PMC article.
-
The Annexin A2/S100A10 Complex: The Mutualistic Symbiosis of Two Distinct Proteins.Biomolecules. 2021 Dec 9;11(12):1849. doi: 10.3390/biom11121849. Biomolecules. 2021. PMID: 34944495 Free PMC article. Review.
-
On the contribution of S100A10 and annexin A2 to plasminogen activation and oncogenesis: an enduring ambiguity.Future Oncol. 2014 Dec;10(15):2469-79. doi: 10.2217/fon.14.163. Future Oncol. 2014. PMID: 25525855 Review.
Cited by
-
Annexin A2 depletion exacerbates the intracerebral microhemorrhage induced by acute rickettsia and Ebola virus infections.PLoS Negl Trop Dis. 2020 Jul 20;14(7):e0007960. doi: 10.1371/journal.pntd.0007960. eCollection 2020 Jul. PLoS Negl Trop Dis. 2020. PMID: 32687500 Free PMC article.
-
Receptor role of the annexin A2 in the mesothelial endocytosis of crocidolite fibers.Lab Invest. 2015 Jul;95(7):749-64. doi: 10.1038/labinvest.2015.28. Epub 2015 Apr 27. Lab Invest. 2015. PMID: 25915724
-
Dysregulated hemostasis in acute promyelocytic leukemia.Int J Hematol. 2024 May;119(5):526-531. doi: 10.1007/s12185-024-03708-0. Epub 2024 Feb 11. Int J Hematol. 2024. PMID: 38341391 Review.
-
Annexin A protein family: Focusing on the occurrence, progression and treatment of cancer.Front Cell Dev Biol. 2023 Mar 3;11:1141331. doi: 10.3389/fcell.2023.1141331. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36936694 Free PMC article. Review.
-
S100A10 and Cancer Hallmarks: Structure, Functions, and its Emerging Role in Ovarian Cancer.Int J Mol Sci. 2018 Dec 19;19(12):4122. doi: 10.3390/ijms19124122. Int J Mol Sci. 2018. PMID: 30572596 Free PMC article. Review.
References
-
- Clark G.B., Morgan R.O., Fernandez M.P., Roux S.J. Evolutionary adaptation of plant annexins has diversified their molecular structures, interactions and functional roles. New Phytol. 2012;196:695–712. - PubMed
-
- Gerke V., Creutz C.E., Moss S.E. Annexins: Linking Ca2+ signalling to membrane dynamics. Nat. Rev. Mol. Cell Biol. 2005;6:449–461. - PubMed
-
- Gerke V., Moss S.E. Annexins: From structure to function. Physiol. Rev. 2002;82:331–371. - PubMed
-
- Seaton B.A., Dedman J.R. Annexins. Biometals. 1998;11:399–404. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

