Evaluation of the roles played by Hcp and VgrG type 6 secretion system effectors in Aeromonas hydrophila SSU pathogenesis
- PMID: 23519162
- PMCID: PMC3709694
- DOI: 10.1099/mic.0.063495-0
Evaluation of the roles played by Hcp and VgrG type 6 secretion system effectors in Aeromonas hydrophila SSU pathogenesis
Abstract
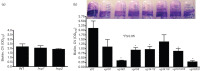
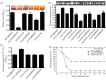
Aeromonas hydrophila, a Gram-negative bacterium, is an emerging human pathogen equipped with both a type 3 and a type 6 secretion system (T6SS). In this study, we evaluated the roles played by paralogous T6SS effector proteins, hemolysin co-regulated proteins (Hcp-1 and -2) and valine glycine repeat G (VgrG-1, -2 and -3) protein family members in A. hydrophila SSU pathogenesis by generating various combinations of deletion mutants of the their genes. In addition to their predicted roles as structural components and effector proteins of the T6SS, our data clearly demonstrated that paralogues of Hcp and VgrG also influenced bacterial motility, protease production and biofilm formation. Surprisingly, there was limited to no observed functional redundancy among and/or between the aforementioned T6SS effector paralogues in multiple assays. Our data indicated that Hcp and VgrG paralogues located within the T6SS cluster were more involved in forming T6SS structures, while the primary roles of Hcp-1 and VgrG-1, located outside of the T6SS cluster, were as T6SS effectors. In terms of influence on bacterial physiology, Hcp-1, but not Hcp-2, influenced bacterial motility and protease production, and in its absence, increases in both of the aforementioned activities were observed. Likewise, VgrG-1 played a major role in regulating bacterial protease production, while VgrG-2 and VgrG-3 were critical in regulating bacterial motility and biofilm formation. In an intraperitoneal murine model of infection, all Hcp and VgrG paralogues were required for optimal bacterial virulence and dissemination to mouse peripheral organs. Importantly, the observed phenotypic alterations of the T6SS mutants could be fully complemented. Taking these results together, we have further established the roles played by the two known T6SS effectors of A. hydrophila by defining their contributions to T6SS function and virulence in both in vitro and in vivo models of infection.
Figures







References
-
- Altwegg M., Martinetti Lucchini G., Lüthy-Hottenstein J., Rohrbach M. (1991). Aeromonas-associated gastroenteritis after consumption of contaminated shrimp. Eur J Clin Microbiol Infect Dis 10, 44–45. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

