G protein signaling in the parasite Entamoeba histolytica
- PMID: 23519208
- PMCID: PMC3641396
- DOI: 10.1038/emm.2013.30
G protein signaling in the parasite Entamoeba histolytica
Abstract
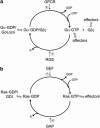
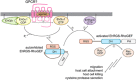
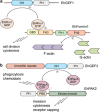
The parasite Entamoeba histolytica causes amebic colitis and systemic amebiasis. Among the known amebic factors contributing to pathogenesis are signaling pathways involving heterotrimeric and Ras superfamily G proteins. Here, we review the current knowledge of the roles of heterotrimeric G protein subunits, Ras, Rho and Rab GTPase families in E. histolytica pathogenesis, as well as of their downstream signaling effectors and nucleotide cycle regulators. Heterotrimeric G protein signaling likely modulates amebic motility and attachment to and killing of host cells, in part through activation of an RGS-RhoGEF (regulator of G protein signaling-Rho guanine nucleotide exchange factor) effector. Rho family GTPases, as well as RhoGEFs and Rho effectors (formins and p21-activated kinases) regulate the dynamic actin cytoskeleton of E. histolytica and associated pathogenesis-related cellular processes, such as migration, invasion, phagocytosis and evasion of the host immune response by surface receptor capping. A remarkably large family of 91 Rab GTPases has multiple roles in a complex amebic vesicular trafficking system required for phagocytosis and pinocytosis and secretion of known virulence factors, such as amebapores and cysteine proteases. Although much remains to be discovered, recent studies of G protein signaling in E. histolytica have enhanced our understanding of parasitic pathogenesis and have also highlighted possible targets for pharmacological manipulation.
Figures
References
-
- Ravdin JI. Amoebiasis. Imperial College Press: London; 2000.
-
- WHO WHO/PAHO/UNESCO report. A consultation with experts on amoebiasis. Mexico City, Mexico 28–29 January, 1997. Epidemiol Bull. 1997;18:13–14. - PubMed
-
- Haque R, Huston CD, Hughes M, Houpt E, Petri WA. Amebiasis. N Engl J Med. 2003;348:1565–1573. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
