Benchmarking CIN 3+ risk as the basis for incorporating HPV and Pap cotesting into cervical screening and management guidelines
- PMID: 23519302
- PMCID: PMC3616419
- DOI: 10.1097/LGT.0b013e318285423c
Benchmarking CIN 3+ risk as the basis for incorporating HPV and Pap cotesting into cervical screening and management guidelines
Abstract
Objective: In 2012, the US Preventive Services Task Force (USPSTF) and a consensus of 25 organizations endorsed concurrent cytology and human papillomavirus (HPV) testing ("cotesting") for cervical cancer screening. Past screening and management guidelines were implicitly based on risks defined by Pap-alone, without consideration of HPV test results. To promote management that is consistent with accepted practice, new guidelines incorporating cotesting should aim to achieve equal management of women at equal risk of cervical intraepithelial neoplasia grade 3 and cancer (CIN 3+).
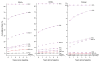
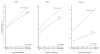
Methods: We estimated cumulative 5-year risks of CIN 3+ for 965,360 women aged 30 to 64 years undergoing cotesting at Kaiser Permanente Northern California over 2003 to 2010. We calculated the implicit risk thresholds for Pap-alone and applied them for new management guidance on HPV and Pap cotesting, citing 2 examples: HPV-positive/atypical squamous cells of undetermined significance (ASC-US) and HPV-negative/Pap-negative. We call this guidance process "benchmarking."
Results: A low-grade squamous intraepithelial lesion result, for which immediate colposcopy is prescribed, carries a 5-year CIN 3+ risk of 5.2%, suggesting that test results with similar risks should be managed with colposcopy. Similarly, ASC-US (2.6% risk) is managed with a 6- to 12-month follow-up visit and Pap-negative (0.26% risk) is managed with a 3-year follow-up visit. The 5-year CIN 3+ risk for women with HPV-positive/ASC-US was 6.8% (95% confidence interval = 6.2%-7.6%). This is greater than the 5.2% risk implicitly leading to referral to colposcopy, consistent with current management recommendations that HPV-positive/ASC-US should be referred for immediate colposcopy. The 5-year CIN 3+ risk for women with HPV-negative/Pap-negative was 0.08% (95% confidence interval = 0.07%-0.09%), far below the 0.26% implicitly required for a 3-year return and justifying a longer (e.g., 5-year) return.
Conclusions: Using the principle of "equal management of equal risks," benchmarking to implicit risk thresholds based on Pap-alone can be used to achieve safe and consistent incorporation of cotesting.
Conflict of interest statement
Figures
References
-
- Moyer VA. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156(12):880–91. W312. - PubMed
-
- Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain JM, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. J Low Genit Tract Dis. 2012;16(3):175–204. - PMC - PubMed
-
- Castle PE, Sideri M, Jeronimo J, Solomon D, Schiffman M. Risk assessment to guide the prevention of cervical cancer. J Low Genit Tract Dis. 2008;12(1):1–7. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

