Five-year risk of recurrence after treatment of CIN 2, CIN 3, or AIS: performance of HPV and Pap cotesting in posttreatment management
- PMID: 23519309
- PMCID: PMC3616418
- DOI: 10.1097/LGT.0b013e31828543c5
Five-year risk of recurrence after treatment of CIN 2, CIN 3, or AIS: performance of HPV and Pap cotesting in posttreatment management
Abstract
Objective: After excisional treatment, cervical intraepithelial neoplasia grade 2 or worse (CIN 2+) can recur. It is not clear how many negative posttreatment Pap or cotest results are needed to ensure adequate safety against CIN 2+ before returning to extended retesting intervals.
Methods: We observed 5-year risks of CIN 2+ for 3 follow-up management strategies after treatment (Pap-alone, human papillomavirus [HPV]-alone, and HPV/Pap cotesting) for 3,273 women aged 25 years and older who were treated for CIN 2, CIN 3, or adenocarcinoma in situ (AIS) between 2003 and 2010 at Kaiser Permanente Northern California.
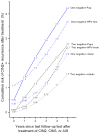
Results: Five-year risks of recurrent CIN 2+ after treatment varied both by antecedent screening test result and the histology of the treated lesion. The risk ranged from 5% for CIN 2 preceded by HPV-positive/atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion to 16% for CIN 3/AIS preceded by atypical glandular cells (AGC)/atypical squamous cells cannot rule out high-grade squamous intraepithelial lesion (ASC-H)/high-grade squamous intraepithelial lesion or worse (HSIL+) (p < .0001). However, after posttreatment negative tests, risks were lowered and similar regardless of antecedent screening test and histology of treated disease. The 5-year recurrent CIN 2+ risk after a negative posttreatment cotest (2.4%) was lower than that following a negative HPV test (3.7%, p = .3) or negative Pap result (4.2%, p = .1). Two negative posttreatment tests of each kind conferred slightly lower 5-year CIN 2+ risk than one (2 negative Pap tests vs. 1, 2.7% vs 4.2%, p = .2; 2 negative HPV tests vs. 1, 2.7% vs 3.7%, p = .7; 2 negative cotests vs. 1, 1.5% vs 2.4%, p = .8). The 5-year CIN 2+ risk after 2 negative cotests of 1.5% (95% confidence interval = 0.3%-7.2%) approached the 0.68% risk after a negative Pap test during routine screening.
Conclusions: Women with antecedent AGC/ASC-H/HSIL+ Pap results or those treated for CIN 3/AIS had a substantial risk of developing CIN 2+ posttreatment. On the basis of the principle of "equal management of equal risks," after negative test results posttreatment, no subgroup of women achieved risk sufficiently low to return to 5-year routine screening. However, negative cotests after treatment provided more reassurance against recurrent CIN 2+ than either negative Pap tests or HPV tests alone.
Conflict of interest statement
Figures


References
-
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. - PubMed
-
- Kocken M, Helmerhorst TJM, Berkhof J, Louwers JA, Nobbenhuis MAE, Bais AG, et al. Risk of recurrent high-grade cervical intraepithelial neoplasia after successful treatment: a long-term multi-cohort study. Lancet Oncol. 2011;12(5):441–50. - PubMed
-
- Soutter WP, de Barros Lopes A, Fletcher A, Monaghan JM, Duncan ID, Paraskevaidis E, et al. Invasive cervical cancer after conservative therapy for cervical intraepithelial neoplasia. Lancet. 1997;349(9057):978–80. - PubMed
-
- Paraskevaidis E, Arbyn M, Sotiriadis A, Diakomanolis E, Martin-Hirsch P, Koliopoulos G, et al. The role of HPV DNA testing in the follow-up period after treatment for CIN: a systematic review of the literature. Cancer Treat Rev. 2004;30(2):205–11. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

