The constant beat: cardiomyocytes adapt their forces by equal contraction upon environmental stiffening
- PMID: 23519595
- PMCID: PMC3603417
- DOI: 10.1242/bio.20133830
The constant beat: cardiomyocytes adapt their forces by equal contraction upon environmental stiffening
Abstract
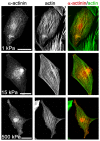
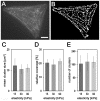
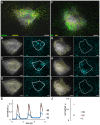
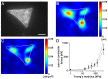
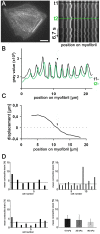
Cardiomyocytes are responsible for the permanent blood flow by coordinated heart contractions. This vital function is accomplished over a long period of time with almost the same performance, although heart properties, as its elasticity, change drastically upon aging or as a result of diseases like myocardial infarction. In this paper we have analyzed late rat embryonic heart muscle cells' morphology, sarcomere/costamere formation and force generation patterns on substrates of various elasticities ranging from ∼1 to 500 kPa, which covers physiological and pathological heart stiffnesses. Furthermore, adhesion behaviour, as well as single myofibril/sarcomere contraction patterns, was characterized with high spatial resolution in the range of physiological stiffnesses (15 kPa to 90 kPa). Here, sarcomere units generate an almost stable contraction of ∼4%. On stiffened substrates the contraction amplitude remains stable, which in turn leads to increased force levels allowing cells to adapt almost instantaneously to changing environmental stiffness. Furthermore, our data strongly indicate specific adhesion to flat substrates via both costameric and focal adhesions. The general appearance of the contractile and adhesion apparatus remains almost unaffected by substrate stiffness.
Keywords: Cardiomyocyte; Cell adhesion; Mechanoresponse; Myofibril; Sarcomere; Traction force microscopy.
Conflict of interest statement
Figures



References
-
- Balaban N. Q., Schwarz U. S., Riveline D., Goichberg P., Tzur G., Sabanay I., Mahalu D., Safran S., Bershadsky A., Addadi L.et al. (2001). Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 3, 466–472 10.1038/35074532 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

