Nonsense and sense suppression abilities of original and derivative Methanosarcina mazei pyrrolysyl-tRNA synthetase-tRNA(Pyl) pairs in the Escherichia coli BL21(DE3) cell strain
- PMID: 23520461
- PMCID: PMC3592851
- DOI: 10.1371/journal.pone.0057035
Nonsense and sense suppression abilities of original and derivative Methanosarcina mazei pyrrolysyl-tRNA synthetase-tRNA(Pyl) pairs in the Escherichia coli BL21(DE3) cell strain
Abstract
Systematic studies of nonsense and sense suppression of the original and three derivative Methanosarcina mazei PylRS-tRNA(Pyl) pairs and cross recognition between nonsense codons and various tRNA(Pyl) anticodons in the Escherichia coli BL21(DE3) cell strain are reported. tRNA(CUA)(Pyl) is orthogonal in E. coli and able to induce strong amber suppression when it is co-expressed with pyrrolysyl-tRNA synthetase (PylRS) and charged with a PylRS substrate, N(ε)-tert-butoxycarbonyl-L-lysine (BocK). Similar to tRNA(CUA)(Pyl), tRNA(UUA)(Pyl) is also orthogonal in E. coli and can be coupled with PylRS to genetically incorporate BocK at an ochre mutation site. Although tRNA(UUA)(Pyl) is expected to recognize a UAG codon based on the wobble hypothesis, the PylRS-tRNA(UUA)(Pyl) pair does not give rise to amber suppression that surpasses the basal amber suppression level in E. coli. E. coli itself displays a relatively high opal suppression level and tryptophan (Trp) is incorporated at an opal mutation site. Although the PylRS-tRNA(UCA)(Pyl) pair can be used to encode BocK at an opal codon, the pair fails to suppress the incorporation of Trp at the same site. tRNA(CCU)(Pyl) fails to deliver BocK at an AGG codon when co-expressed with PylRS in E. coli.
Conflict of interest statement
Figures
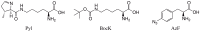







Similar articles
-
Recognition of non-alpha-amino substrates by pyrrolysyl-tRNA synthetase.J Mol Biol. 2009 Feb 6;385(5):1352-60. doi: 10.1016/j.jmb.2008.11.059. Epub 2008 Dec 11. J Mol Biol. 2009. PMID: 19100747
-
Mutually orthogonal pyrrolysyl-tRNA synthetase/tRNA pairs.Nat Chem. 2018 Aug;10(8):831-837. doi: 10.1038/s41557-018-0052-5. Epub 2018 May 28. Nat Chem. 2018. PMID: 29807989 Free PMC article.
-
An Evolved Methanomethylophilus alvus Pyrrolysyl-tRNA Synthetase/tRNA Pair Is Highly Active and Orthogonal in Mammalian Cells.Biochemistry. 2019 Feb 5;58(5):387-390. doi: 10.1021/acs.biochem.8b00808. Epub 2018 Sep 27. Biochemistry. 2019. PMID: 30260626 Free PMC article.
-
tRNAPyl: Structure, function, and applications.RNA Biol. 2018;15(4-5):441-452. doi: 10.1080/15476286.2017.1356561. Epub 2017 Sep 13. RNA Biol. 2018. PMID: 28837402 Free PMC article. Review.
-
Pyrrolysyl-tRNA synthetase: an ordinary enzyme but an outstanding genetic code expansion tool.Biochim Biophys Acta. 2014 Jun;1844(6):1059-70. doi: 10.1016/j.bbapap.2014.03.002. Epub 2014 Mar 12. Biochim Biophys Acta. 2014. PMID: 24631543 Free PMC article. Review.
Cited by
-
Directed Evolution of Methanomethylophilus alvus Pyrrolysyl-tRNA Synthetase Generates a Hyperactive and Highly Selective Variant.Front Mol Biosci. 2022 Mar 9;9:850613. doi: 10.3389/fmolb.2022.850613. eCollection 2022. Front Mol Biosci. 2022. PMID: 35372501 Free PMC article.
-
Pyrrolysyl-tRNA synthetase, an aminoacyl-tRNA synthetase for genetic code expansion.Croat Chem Acta. 2016 Jun;89(2):163-174. doi: 10.5562/cca2825. Epub 2016 Jun 14. Croat Chem Acta. 2016. PMID: 28239189 Free PMC article.
-
Efficient suppression of premature termination codons with alanine by engineered chimeric pyrrolysine tRNAs.Nucleic Acids Res. 2024 Dec 11;52(22):14244-14259. doi: 10.1093/nar/gkae1048. Nucleic Acids Res. 2024. PMID: 39558163 Free PMC article.
-
Evolution of translation machinery in recoded bacteria enables multi-site incorporation of nonstandard amino acids.Nat Biotechnol. 2015 Dec;33(12):1272-1279. doi: 10.1038/nbt.3372. Epub 2015 Nov 16. Nat Biotechnol. 2015. PMID: 26571098 Free PMC article.
-
Development of orthogonal aminoacyl-tRNA synthetase mutant for incorporating a non-canonical amino acid.AMB Express. 2024 May 24;14(1):60. doi: 10.1186/s13568-024-01706-3. AMB Express. 2024. PMID: 38782816 Free PMC article.
References
-
- Hao B, Gong W, Ferguson TK, James CM, Krzycki JA, et al. (2002) A New UAG-Encoded Residue in the Structure of a Methanogen Methyltransferase. Science 296: 1462–1466. - PubMed
-
- Srinivasan G, James CM, Krzycki JA (2002) Pyrrolysine Encoded by UAG in Archaea: Charging of a UAG-Decoding Specialized tRNA. Science 296: 1459–1462. - PubMed
-
- Baron C, Böck A (1991) The length of the aminoacyl-acceptor stem of the selenocysteine-specific tRNA(Sec) of Escherichia coli is the determinant for binding to elongation factors SELB or Tu. J Biol Chem 266: 20375–20379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

