AQP4-dependent water transport plays a functional role in exercise-induced skeletal muscle adaptations
- PMID: 23520529
- PMCID: PMC3592820
- DOI: 10.1371/journal.pone.0058712
AQP4-dependent water transport plays a functional role in exercise-induced skeletal muscle adaptations
Erratum in
- PLoS One. 2013;8(6). doi:10.1371/annotation/86fc2632-913c-490d-8b9b-e925b38baec5
Abstract
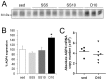
In this study we assess the functional role of Aquaporin-4 (AQP4) in the skeletal muscle by analyzing whether physical activity modulates AQP4 expression and whether the absence of AQP4 has an effect on osmotic behavior, muscle contractile properties, and physical activity. To this purpose, rats and mice were trained on the treadmill for 10 (D10) and 30 (D30) days and tested with exercise to exhaustion, and muscles were used for immunoblotting, RT-PCR, and fiber-type distribution analysis. Taking advantage of the AQP4 KO murine model, functional analysis of AQP4 was performed on dissected muscle fibers and sarcolemma vesicles. Moreover, WT and AQP4 KO mice were subjected to both voluntary and forced activity. Rat fast-twitch muscles showed a twofold increase in AQP4 protein in D10 and D30 rats compared to sedentary rats. Such increase positively correlated with the animal performance, since highest level of AQP4 protein was found in high runner rats. Interestingly, no shift in muscle fiber composition nor an increase in AQP4-positive fibers was found. Furthermore, no changes in AQP4 mRNA after exercise were detected, suggesting that post-translational events are likely to be responsible for AQP4 modulation. Experiments performed on AQP4 KO mice revealed a strong impairment in osmotic responses as well as in forced and voluntary activities compared to WT mice, even though force development amplitude and contractile properties were unvaried. Our findings definitively demonstrate the physiological role of AQP4 in supporting muscle contractile activity and metabolic changes that occur in fast-twitch skeletal muscle during prolonged exercise.
Conflict of interest statement
Figures







References
-
- Usher-Smith JA, Huang CL, Fraser JA (2009) Control of cell volume in skeletal muscle. Biol Rev Camb Philos Soc 84: 143–159. - PubMed
-
- Frigeri A, Gropper MA, Umenishi F, Kawashima M, Brown D, et al. (1995) Localization of MIWC and GLIP water channel homologs in neuromuscular, epithelial and glandular tissues. J Cell Sci 108 ( Pt 9): 2993–3002. - PubMed
-
- Verbavatz JM, Ma T, Gobin R, Verkman AS (1997) Absence of orthogonal arrays in kidney, brain and muscle from transgenic knockout mice lacking water channel aquaporin-4. J Cell Sci 110 ( Pt 22): 2855–2860. - PubMed
-
- Silberstein C, Bouley R, Huang Y, Fang P, Pastor-Soler N, et al. (2004) Membrane organization and function of M1 and M23 isoforms of aquaporin-4 in epithelial cells. Am J Physiol Renal Physiol 287: F501–511. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

