Gli1 deletion prevents Helicobacter-induced gastric metaplasia and expansion of myeloid cell subsets
- PMID: 23520544
- PMCID: PMC3592845
- DOI: 10.1371/journal.pone.0058935
Gli1 deletion prevents Helicobacter-induced gastric metaplasia and expansion of myeloid cell subsets
Abstract
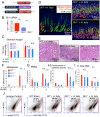
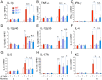
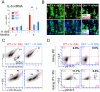
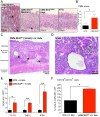
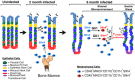
Chronic inflammation in the stomach induces metaplasia, the pre-cancerous lesion that precedes inflammation-driven neoplastic transformation. While Hedgehog signaling contributes to the initiation of some cancers, its role in gastric transformation remains poorly defined. We found that Helicobacter-infected C57BL/6 mice develop extensive mucous cell metaplasia at 6 month but not at 2 months post-infection. Gastric metaplasia coincided with the appearance of CD45(+)MHCII(+)CD11b(+)CD11c(+) myeloid cells that were normally not present in the chronic gastritis at 2 months. The myeloid regulatory gene Schlafen-4 was identified in a microarray analysis comparing infected WT versus Gli1 null mice and was expressed in the CD11b(+)CD11c(+) myeloid population. Moreover this same population expressed IL-1β and TNFα pro-inflammatory cytokines. By 6 months, the mucous neck cell metaplasia (SPEM) expressed IL-6, phosphorylated STAT3 and the proliferative marker Ki67. Expression was not observed in Gli1 mutant mice consistent with the requirement of Gli1 to induce this pre-neoplastic phenotype. Ectopic Shh ligand expression alone was not sufficient to induce SPEM, but with Helicobacter infection synergistically increased the histologic severity observed with the inflammation. Therefore Hedgehog signaling is required, but is not sufficient to generate pre-neoplastic changes during chronic gastritis. Gli1-dependent myeloid cell differentiation plays a pivotal role in the appearance of myeloid cell subtypes ostensibly required for SPEM development. Moreover, it suggests that therapies capable of targeting this phenotypic switch might prevent progression to metaplasia, the pre-neoplastic change that develops prior to dysplasia and gastric cancer, which also occurs in other epithelial-derived neoplasias initiated by chronic inflammation.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

