Analysis of telomere length and function in radiosensitive mouse and human cells in response to DNA-PKcs inhibition
- PMID: 23521760
- PMCID: PMC3614538
- DOI: 10.1186/2041-9414-4-2
Analysis of telomere length and function in radiosensitive mouse and human cells in response to DNA-PKcs inhibition
Abstract
Background: Telomeres, the physical ends of chromosomes, play an important role in preserving genomic integrity. This protection is supported by telomere binding proteins collectively known as the shelterin complex. The shelterin complex protects chromosome ends by suppressing DNA damage response and acting as a regulator of telomere length maintenance by telomerase, an enzyme that elongates telomeres. Telomere dysfunction manifests in different forms including chromosomal end-to-end fusion, telomere shortening and p53-dependent apoptosis and/or senescence. An important shelterin-associated protein with critical role in telomere protection in human and mouse cells is the catalytic subunit of DNA-protein kinase (DNA-PKcs). DNA-PKcs deficiency in mouse cells results in elevated levels of spontaneous telomeric fusion, a marker of telomere dysfunction, but does not cause telomere length shortening. Similarly, inhibition of DNA-PKcs with chemical inhibitor, IC86621, prevents chromosomal end protection through mechanism reminiscent of dominant-negative reduction in DNA-PKcs activity.
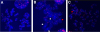
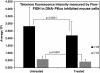
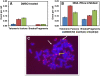
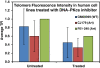
Results: We demonstrate here that the IC86621 mediated inhibition of DNA-PKcs in two mouse lymphoma cell lines results not only in elevated frequencies of chromosome end-to-end fusions, but also accelerated telomere shortening in the presence of telomerase. Furthermore, we observed increased levels of spontaneous telomeric fusions in Artemis defective human primary fibroblasts in which DNA-PKcs was inhibited, but no significant changes in telomere length.
Conclusion: These results confirm that DNA-PKcs plays an active role in chromosome end protection in mouse and human cells. Furthermore, it appears that DNA-PKcs is also involved in telomere length regulation, independently of telomerase activity, in mouse lymphoma cells but not in human cells.
Figures
Similar articles
-
DNA-dependent protein kinase in telomere maintenance and protection.Cell Mol Biol Lett. 2020 Jan 17;25:2. doi: 10.1186/s11658-020-0199-0. eCollection 2020. Cell Mol Biol Lett. 2020. PMID: 31988640 Free PMC article. Review.
-
DNA-dependent protein kinase catalytic subunit is not required for dysfunctional telomere fusion and checkpoint response in the telomerase-deficient mouse.Mol Cell Biol. 2007 Mar;27(6):2253-65. doi: 10.1128/MCB.01354-06. Epub 2006 Dec 4. Mol Cell Biol. 2007. PMID: 17145779 Free PMC article.
-
Defective Artemis causes mild telomere dysfunction.Genome Integr. 2010 May 26;1(1):3. doi: 10.1186/2041-9414-1-3. Genome Integr. 2010. PMID: 20678254 Free PMC article.
-
Functional interaction between DNA-PKcs and telomerase in telomere length maintenance.EMBO J. 2002 Nov 15;21(22):6275-87. doi: 10.1093/emboj/cdf593. EMBO J. 2002. PMID: 12426399 Free PMC article.
-
DNA repair factors and telomere-chromosome integrity in mammalian cells.Cytogenet Genome Res. 2004;104(1-4):116-22. doi: 10.1159/000077475. Cytogenet Genome Res. 2004. PMID: 15162024 Review.
Cited by
-
DNA-dependent protein kinase in telomere maintenance and protection.Cell Mol Biol Lett. 2020 Jan 17;25:2. doi: 10.1186/s11658-020-0199-0. eCollection 2020. Cell Mol Biol Lett. 2020. PMID: 31988640 Free PMC article. Review.
-
DNA Damage Signalling and Repair Inhibitors: The Long-Sought-After Achilles' Heel of Cancer.Biomolecules. 2015 Nov 20;5(4):3204-59. doi: 10.3390/biom5043204. Biomolecules. 2015. PMID: 26610585 Free PMC article. Review.
-
Crosstalk between telomere maintenance and radiation effects: A key player in the process of radiation-induced carcinogenesis.Mutat Res Rev Mutat Res. 2014 Jan 31:S1383-5742(14)00002-7 10.1016/j.mrrev.2014.01.001. doi: 10.1016/j.mrrev.2014.01.001. Online ahead of print. Mutat Res Rev Mutat Res. 2014. PMID: 24486376 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
