FMR1, circadian genes and depression: suggestive associations or false discovery?
- PMID: 23521777
- PMCID: PMC3627611
- DOI: 10.1186/1740-3391-11-3
FMR1, circadian genes and depression: suggestive associations or false discovery?
Abstract
Background: There are several indications that malfunctions of the circadian clock contribute to depression. To search for particular circadian gene polymorphisms associated with depression, diverse polymorphisms were genotyped in two samples covering a range of depressed volunteers and participants with normal mood.
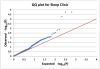
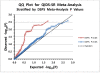
Methods: Depression mood self-ratings and DNA were collected independently from a sample of patients presenting to a sleep disorders center (1086 of European origin) and from a separate sample consisting of 399 participants claiming delayed sleep phase symptoms and 406 partly-matched controls. A custom Illumina Golden Gate array of 768 selected single nucleotide polymorphisms (SNPs) was assayed in both samples, supplemented by additional SNPlex and Taqman assays, including assay of 41 ancestry-associated markers (AIMs) to control stratification.
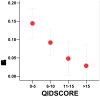
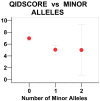
Results: In the Sleep Clinic sample, these assays yielded Bonferroni-significant association with depressed mood in three linked SNPs of the gene FMR1: rs25702 (nominal P=1.77E-05), rs25714 (P=1.83E-05), and rs28900 (P=5.24E-05). This FMR1 association was supported by 8 SNPs with nominal significance and a nominally-significant gene-wise set test. There was no association of depressed mood with FMR1 in the delayed sleep phase case-control sample or in downloaded GWAS data from the GenRED 2 sample contrasting an early-onset recurrent depression sample with controls. No replication was located in other GWAS studies of depression. Our data did weakly replicate a previously-reported association of depression with PPARGC1B rs7732671 (P=0.0235). Suggestive associations not meeting strict criteria for multiple testing and replication were found with GSK3B, NPAS2, RORA, PER3, CRY1, MTNR1A and NR1D1. Notably, 16 SNPs nominally associated with depressed mood (14 in GSK3B) were also nominally associated with delayed sleep phase syndrome (P=3E10-6).
Conclusions: Considering the inconsistencies between samples and the likelihood that the significant three FMR1 SNPs might be linked to complex polymorphisms more functionally related to depression, large gene resequencing studies may be needed to clarify the import for depression of these circadian genes.
Figures
Similar articles
-
Circadian polymorphisms associated with affective disorders.J Circadian Rhythms. 2009 Jan 23;7:2. doi: 10.1186/1740-3391-7-2. J Circadian Rhythms. 2009. PMID: 19166596 Free PMC article.
-
Differential association of circadian genes with mood disorders: CRY1 and NPAS2 are associated with unipolar major depression and CLOCK and VIP with bipolar disorder.Neuropsychopharmacology. 2010 May;35(6):1279-89. doi: 10.1038/npp.2009.230. Epub 2010 Jan 13. Neuropsychopharmacology. 2010. PMID: 20072116 Free PMC article.
-
Effects of circadian clock genes and health-related behavior on metabolic syndrome in a Taiwanese population: Evidence from association and interaction analysis.PLoS One. 2017 Mar 15;12(3):e0173861. doi: 10.1371/journal.pone.0173861. eCollection 2017. PLoS One. 2017. PMID: 28296937 Free PMC article.
-
[Circadian markers and genes in bipolar disorder].Encephale. 2015 Sep;41(4 Suppl 1):S38-44. doi: 10.1016/S0013-7006(15)30005-1. Encephale. 2015. PMID: 26746321 Review. French.
-
Mechanisms of rapid antidepressant effects of sleep deprivation therapy: clock genes and circadian rhythms.Biol Psychiatry. 2013 Jun 15;73(12):1164-71. doi: 10.1016/j.biopsych.2012.07.020. Epub 2012 Aug 18. Biol Psychiatry. 2013. PMID: 22906517 Review.
Cited by
-
Circadian polymorphisms in night owls, in bipolars, and in non-24-hour sleep cycles.Psychiatry Investig. 2014 Oct;11(4):345-62. doi: 10.4306/pi.2014.11.4.345. Epub 2014 Oct 20. Psychiatry Investig. 2014. PMID: 25395965 Free PMC article.
-
Genetics and epigenetics of circadian rhythms and their potential roles in neuropsychiatric disorders.Neurosci Bull. 2015 Feb;31(1):141-59. doi: 10.1007/s12264-014-1495-3. Epub 2015 Feb 6. Neurosci Bull. 2015. PMID: 25652815 Free PMC article. Review.
-
Evaluation of the Association Between Genetic Variants in Circadian Rhythm Genes and Posttraumatic Stress Symptoms Identifies a Potential Functional Allele in the Transcription Factor TEF.Front Psychiatry. 2018 Nov 15;9:597. doi: 10.3389/fpsyt.2018.00597. eCollection 2018. Front Psychiatry. 2018. PMID: 30498461 Free PMC article.
-
Integrated Analysis of microRNA and mRNA Expression Profiles: An Attempt to Disentangle the Complex Interaction Network in Attention Deficit Hyperactivity Disorder.Brain Sci. 2019 Oct 22;9(10):288. doi: 10.3390/brainsci9100288. Brain Sci. 2019. PMID: 31652596 Free PMC article.
-
Construction and dissection of the ceRNA‑ceRNA network reveals critical modules in depression.Mol Med Rep. 2019 May;19(5):3411-3420. doi: 10.3892/mmr.2019.10009. Epub 2019 Mar 5. Mol Med Rep. 2019. PMID: 30864711 Free PMC article.
References
-
- Kripke DF, Mullaney DJ, Atkinson M, Wolf S. Circadian rhythm disorders in manic-depressives. Biol Psychiatry. 1978;13:335–351. - PubMed
-
- Halberg F. Symposium Bell-Air III. Geneva: Mason et Cie; 1967. Physiologic considerations underlying rhythmometry, with special reference to emotional illness. Symposium on biological cycles and psychiatry; pp. 73–126.
-
- Hodgson K, McGuffin P. The Genetic Basis of Depression. Behav Neurosci: Curr Top; 2013. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

