The combination of ANT2 shRNA and hNIS radioiodine gene therapy increases CTL cytotoxic activity through the phenotypic modulation of cancer cells: combination treatment with ANT2 shRNA and I-131
- PMID: 23522027
- PMCID: PMC3653759
- DOI: 10.1186/1471-2407-13-143
The combination of ANT2 shRNA and hNIS radioiodine gene therapy increases CTL cytotoxic activity through the phenotypic modulation of cancer cells: combination treatment with ANT2 shRNA and I-131
Abstract
Background: It is important to simultaneously induce strong cell death and antitumor immunity in cancer patients for successful cancer treatment. Here, we investigated the cytotoxic and phenotypic modulation effects of the combination of ANT2 shRNA and human sodium iodide symporter (hNIS) radioiodine gene therapy in vitro and in vivo and visualized the antitumor effects in an immunocompromised mouse colon cancer model.
Methods: A mouse colon cancer cell line co-expressing hNIS and the luciferase gene (CT26/hNIS-Fluc, named CT26/NF) was established. CT26/NF cells and tumor-bearing mice were treated with HBSS, scramble, ANT2 shRNA, I-131, and ANT2 shRNA + I-131. The apoptotic rates (%) and MHC class I and Fas gene expression levels were determined in treated CT26/NF cells using flow cytometry. Concurrently, the level of caspase-3 activation was determined in treated cells in vitro. For in vivo therapy, tumor-bearing mice were treated with scramble, ANT2 shRNA, I-131, and the combination therapy, and the anti-tumor effects were monitored using bioluminescence. The killing activity of cytotoxic T cells (CTLs) was measured with a lactate dehydrogenase (LDH) assay.
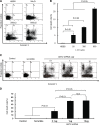
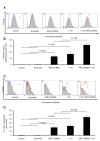
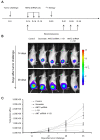
Results: For the in vitro experiments, the combination of ANT2 shRNA and I-131 resulted in a higher apoptotic cell death rate compared with ANT2 shRNA or I-131 alone, and the levels of MHC class I and Fas-expressing cancer cells were highest in the cells receiving combination treatment, while single treatment modestly increased the level of MHC class I and Fas gene expression. The combination of ANT2 shRNA and I-131 resulted in a higher caspase-3 activation than single treatments. Interestingly, in vivo combination treatment led to increased gene expression of MHC class I and Fas than the respective mono-therapies; furthermore, bioluminescence showed increased antitumor effects after combination treatment than monotherapies. The LDH assay revealed that the CTL killing activity against CT26/NF cells was most effective after combination therapy.
Conclusions: Increased cell death and phenotypic modulation of cancer cells in vitro and in vivo were achieved simultaneously after combination therapy with ANT2 shRNA and I-131, and this combination therapy induced remarkable antitumor outcomes through improvements in CTL immunity against CT26/NF. Our results suggest that combination therapy can be used as a new therapeutic strategy for cancer patients who show resistance to single therapy such as radiation or immunotherapy.
Figures



Similar articles
-
Human sodium/iodide symporter-mediated radioiodine gene therapy enhances the killing activities of CTLs in a mouse tumor model.Mol Cancer Ther. 2010 Jan;9(1):126-33. doi: 10.1158/1535-7163.MCT-09-0540. Epub 2010 Jan 6. Mol Cancer Ther. 2010. PMID: 20053774
-
Human sodium iodide symporter added to multidrug resistance 1 small hairpin RNA in a single gene construct enhances the therapeutic effects of radioiodine in a nude mouse model of multidrug resistant colon cancer.Cancer Biother Radiopharm. 2010 Dec;25(6):671-9. doi: 10.1089/cbr.2010.0837. Cancer Biother Radiopharm. 2010. PMID: 21204761
-
Combined RNA interference of adenine nucleotide translocase-2 and ganciclovir therapy in hepatocellular carcinoma.Nucl Med Biol. 2013 Nov;40(8):987-93. doi: 10.1016/j.nucmedbio.2013.08.004. Epub 2013 Sep 17. Nucl Med Biol. 2013. PMID: 24054501
-
In vivo scintigraphic imaging of antitumor effects by combined radioiodine therapy and human sodium iodide symporter gene immunotherapy.Mol Imaging. 2010 Jun;9(3):141-52. Mol Imaging. 2010. PMID: 20487680
-
The mitochondrial adenine nucleotide transporters in myogenesis.Free Radic Biol Med. 2022 Aug 1;188:312-327. doi: 10.1016/j.freeradbiomed.2022.05.022. Epub 2022 Jun 15. Free Radic Biol Med. 2022. PMID: 35714845 Review.
Cited by
-
Stimulation of murine cell-mediated immunity by dietary administration of a cell preparation of Enterococcus faecalis strain KH-2 and its possible activity against tumour development in mice.Biosci Microbiota Food Health. 2018;37(3):49-57. doi: 10.12938/bmfh.17-021. Epub 2018 Mar 28. Biosci Microbiota Food Health. 2018. PMID: 30094120 Free PMC article.
-
Differential Expression of ADP/ATP Carriers as a Biomarker of Metabolic Remodeling and Survival in Kidney Cancers.Biomolecules. 2020 Dec 30;11(1):38. doi: 10.3390/biom11010038. Biomolecules. 2020. PMID: 33396658 Free PMC article.
-
Porcine IL-12 plasmid as an adjuvant improves the cellular and humoral immune responses of DNA vaccine targeting transmissible gastroenteritis virus spike gene in a mouse model.J Vet Med Sci. 2019 Oct 18;81(10):1438-1444. doi: 10.1292/jvms.18-0682. Epub 2019 Sep 2. J Vet Med Sci. 2019. PMID: 31474664 Free PMC article.
-
T cell immune abnormalities in immune thrombocytopenia.J Hematol Oncol. 2014 Oct 2;7:72. doi: 10.1186/s13045-014-0072-6. J Hematol Oncol. 2014. PMID: 25274611 Free PMC article. Review.
-
Role of ANT2 in mitochondrial function and cancer cell survival: a target for therapeutic intervention.Cell Death Discov. 2025 May 8;11(1):225. doi: 10.1038/s41420-025-02510-z. Cell Death Discov. 2025. PMID: 40335504 Free PMC article. No abstract available.
References
-
- Rosenberg SA, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ. Inability to immunize patients with metastatic melanoma using plasmid DNA encoding the gp100 melanoma-melanocyte antigen. Hum Gene Ther. 2003;14(8):709–714. doi: 10.1089/104303403765255110. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

